Moxifloxacin (Bayer’s Avelox) is a fluoroquinolone used for the treatment of respiratory tract infections including acute exacerbations of chronic bronchitis. In December 1999, the FDA approved the oral formulation of moxifloxacin, and in December 2001, the agency approved the parenteral formulation of this product for the same indications. In Germany, oral moxifloxacin received regulatory approval in September 1999 for the treatment of community-acquired infections, including acute exacerbations of chronic bronchitis.
The parenteral formulation was approved in May 2002. Oral moxifloxacin was recently launched in Spain and Italy and is awaiting regulatory approval in other European markets. In Japan, moxifloxacin is in Phase III and Phase I trials for the oral and IV formulations, respectively. This agent will retain patent protection through 2014 in the United States and through 2009 in Europe and Japan. Moxifloxacin displays excellent activity against a broad spectrum of bacteria, including penicillin- and macrolide-resistant S. pneumoniae. It is highly active against the following:
- Common RTI pathogens such as S. pneumoniae, H. influenzae, and M. catarrhalis.
- RTI pathogens with reduced susceptibility to conventional agents such as penicillin and macrolide-resistant strains of S. pneumoniae.
- Unusual community RTI pathogens such as Staphylococcus aureus and K. pneumoniae.
- Atypical microorganisms, such as Mycoplasma, Legionella, and Chlamydia.
Moxifloxacin offers superior activity against S. pneumoniae compared with that of current quinolones (e.g., ciprofloxacin, levofloxacin) and is extremely effective in treating other respiratory pathogens. A multi-center, multinational, randomized, double-blind Phase III study compared moxifloxacin with amoxicillin in patients experiencing acute exacerbations of chronic bronchitis. During a 12-month monitoring period, 730 patients with acute exacerbations of chronic bronchitis were randomized to receive moxifloxacin (400 mg, once daily, for five days) or a standard treatment regimen consisting of amoxicillin (500 mg, three times daily, for seven days) or clarithromycin (500 mg, twice daily, for seven days) or the cephalosporin cefuroxime axetil (250 mg, twice daily, for seven days).
The primary end point was clinical success (resolution and improvement) at 7-10 days post treatment. Patients were also monitored after nine months to assess long-term outcomes, including time until their next exacerbation. Moxifloxacin demonstrated a significantly higher clinical cure rate (70.9% versus 62.8% in the comparator arm) and a significantly higher bacteriological response rate (92% versus 81% in the comparator arm). A follow-up study showed that for patients who received moxifloxacin, the average interval between episodes of acute exacerbations of chronic bronchitis was approximately two weeks longer than for those who received standard therapy. Moxifloxacin is not associated with phototoxicity, but it has been shown to alter the QT interval above baseline. The frequency and severity of adverse reactions were equal to those experienced among the comparators (clarithromycin and cefuroxime axetil) and included nausea (8%), diarrhea (6%), and dizziness (3%).
Uses
Moxifloxacin is used orally or IV for the treatment of respiratory tract infections (acute sinusitis, acute exacerbations of chronic bronchitis, community-acquired pneumonia) and uncomplicated skin and skin structure infections caused by susceptible bacteria. Moxifloxacin also is used as an alternative agent for the treatment of active tuberculosis. In addition, the drug has been recommended as an alternative agent for postexposure prophylaxis following suspected or confirmed exposure to aerosolized anthrax spores (inhalational anthrax) or for treatment of inhalational anthrax.

Respiratory Tract Infections
Moxifloxacin is used for the treatment of acute bacterial sinusitis caused by susceptible Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis; acute bacterial exacerbations of chronic bronchitis caused by susceptible S. pneumoniae, H. influenzae, H. parainfluenzae, Klebsiella pneumoniae, Staphylococcus aureus, or M. catarrhalis; and community-acquired pneumonia (CAP) caused by susceptible S. pneumoniae (including penicillin-resistant strains with penicillin MICs 2 mcg/mL or greater), S. aureus, K. pneumoniae, H. influenzae, Mycoplasma pneumoniae, Chlamydia pneumoniae, or M. catarrhalis.
In a limited number of randomized, comparative clinical trials, rates of clinical success (generally defined as the percentage of patients with clinical cure or improvement) were similar for moxifloxacin (400 mg once daily for 10 days) or cefuroxime axetil (250 mg twice daily for 10 days) in patients with acute bacterial sinusitis; moxifloxacin (400 mg once daily for 5 days) or clarithromycin (500 mg twice daily for 10 days) in patients with acute exacerbations of chronic bronchitis; or moxifloxacin (400 mg once daily for 10 days) or clarithromycin (500 mg twice daily for 10 days) in patients with mild to moderate CAP.
Community-acquired Pneumonia
In a large randomized study in patients with clinically and radiologically documented CAP, the rate of clinical success with a sequential regimen of IV moxifloxacin following by oral moxifloxacin (400 mg daily for 7-14 days) was similar to that achieved with 7-14 days of therapy with similar regimens using other fluoroquinolones (i.e., levofloxacin).
Data on efficacy of moxifloxacin in adults for the treatment of CAP caused by S. pneumoniae has been obtained from 9 clinical studies. In these studies, moxifloxacin resulted in clinical cure in 94% of patients with CAP caused by S. pneumoniae. In those patients with documented infections caused by penicillin-resistant S. pneumoniae (penicillin MICs 2 mcg/mL or greater), the bacteriologic cure rate was 100%.
Some clinicians, including the American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA), suggest that fluoroquinolones with enhanced activity against S. pneumoniae (e.g., gatifloxacin, gemifloxacin, levofloxacin, moxifloxacin) are drugs of choice for the treatment of CAP in adults.
However, there is disagreement over the role of these drugs in the outpatient treatment of CAP. The IDSA recommends monotherapy with these oral fluoroquinolones as one of several possible regimens for empiric outpatient treatment of acute CAP in immunocompetent adults, but the ATS suggests that these agents not be used for outpatient treatment of CAP in immunocompetent adults without cardiopulmonary disease or other modifying factors that would increase the risk of multidrug-resistant S. pneumonia.
To limit emergence of fluoroquinolone-resistant strains, the US Centers for Disease Control and Prevention (CDC) suggests that use of these oral fluoroquinolones in the outpatient treatment of CAP be reserved for when other anti-infectives are ineffective or cannot be used or when highly penicillin-resistant S. pneumoniae (penicillin MICs 2 mcg/mL or greater) are identified as the cause of infection. For further information on treatment of CAP.
Skin and Skin Structure Infections
Moxifloxacin is used for the treatment of uncomplicated skin and skin structure infections caused by susceptible S. aureus or Streptococcus pyogenes (group A b-hemolytic streptococci). The drug has been effective in the treatment of uncomplicated abscesses, furuncles, cellulitis, impetigo, and other skin infections. In a randomized double-blind study, moxifloxacin (400 mg once daily for 7 days) was as effective as cephalexin (500 mg 3 times daily for 7 days) in the treatment of uncomplicated skin and skin structure infections caused by susceptible bacteria; resolution or improvement occurred in 89 or 91% of those receiving moxifloxacin or cephalexin, respectively (intent-to-treat analysis).
Mycobacterial Infections
Treatment of Active Tuberculosis
Fluoroquinolones, including moxifloxacin, have been used in multiple-drug regimens for the treatment of active tuberculosis in patients with infections caused by Mycobacterium tuberculosis resistant to first-line agents and in patients intolerant of some first-line agents.
Although the potential role of fluoroquinolones and the optimal length of therapy have not been fully defined, the CDC, ATS, and IDSA state that use of fluoroquinolones as alternative agents for the treatment of active tuberculosis can be considered in patients with relapse, treatment failure, or M. tuberculosis resistant to isoniazid and/or rifampin or when first-line drugs cannot be tolerated.
It has been theorized that adding a fluoroquinolone to a first-line multiple-drug regimen possibly may enhance the bactericidal efficacy of the regimen, prevent development of resistance, or shorten the duration of treatment needed; however, the CDC, ATS, and IDSA state that fluoroquinolones should not be considered first-line agents for the treatment of tuberculosis caused by M. tuberculosis susceptible to first-line agents.
Although there is clinical experience with several fluoroquinolones in the treatment of tuberculosis, the ATS, CDC, and IDSA recommend use of gatifloxacin, levofloxacin, or moxifloxacin as second-line agents and, on the basis of cumulative experience, these experts suggest that levofloxacin may be the preferred oral fluoroquinolone when use of one of these drugs is considered necessary in the treatment of the disease.
When an alternative regimen is indicated for the treatment of active tuberculosis because of drug resistance or intolerance and when rifampin cannot be used (e.g., because of rifampin-resistant strains), the CDC, ATS, and IDSA suggest that a regimen of isoniazid, ethambutol, and a fluoroquinolone given for 12-18 months (with pyrazinamide given during at least the first 2 months) can be considered.
For the treatment of pulmonary tuberculosis caused by isoniazid-resistant M. tuberculosis (with or without resistance to streptomycin), these experts suggest that adding a fluoroquinolone to a 6-month regimen of rifampin, ethambutol, and pyrazinamide can be considered for patients who have extensive disease. For the treatment of pulmonary tuberculosis caused by isoniazid- and rifampin-resistant strains (with or without resistance to streptomycin), an 18- to 24-month regimen of a fluoroquinolone, ethambutol, pyrazinamide, a parenteral agent (e.g., streptomycin, amikacin, kanamycin, capreomycin) with or without another alternative agent can be considered.
When the infection is caused by strains resistant to isoniazid, rifampin, ethambutol, and/or pyrazinamide (with or without streptomycin resistance), a 24-month regimen of a fluoroquinolone, ethambutol or pyrazinamide (if active), a parenteral agent (e.g., streptomycin, amikacin, kanamycin, capreomycin), and 2 other alternative agents can be considered. The most recent CDC, ATS, and IDSA recommendations for the treatment of tuberculosis should be consulted for more specific information.
Anthrax
The US Working Group on Civilian Biodefense suggests that, based on in vitro data, oral moxifloxacin can be considered an alternative agent for postexposure prophylaxis following suspected or confirmed exposure to aerosolized anthrax spores (inhalational anthrax) when oral ciprofloxacin and oral doxycycline are unavailable. These experts also suggest that oral moxifloxacin can be considered an alternative for the treatment of inhalational anthrax when a parenteral regimen is not available (e.g., when there are supply or logistic problems because large numbers of individuals require treatment in a mass casualty setting).
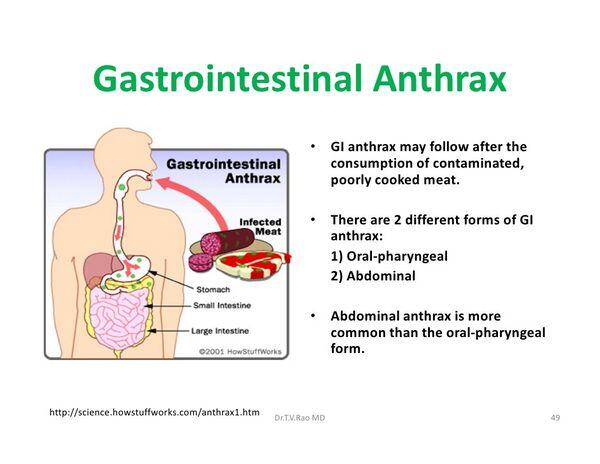
The CDC and other experts (e.g., US Working Group on Civilian Biodefense) recommend that treatment of inhalational anthrax be initiated with a multiple-drug parenteral regimen that includes ciprofloxacin or doxycycline and 1 or 2 other anti-infectives predicted to be effective; however, use of these parenteral regimens may not be possible if large numbers of individuals require treatment in a mass casualty setting and it may be necessary to use an oral regimen.
Although there are no animal or human studies to date evaluating use of moxifloxacin for treatment or prophylaxis of anthrax and fluoroquinolones other than ciprofloxacin currently are not included in CDC’s recommended regimens, in vitro evidence suggests that other fluoroquinolones would be as effective as ciprofloxacin in treating anthrax. For additional information on treatment of anthrax and recommendations for prophylaxis following exposure to anthrax spores.
Ophthalmic Infections
For use of moxifloxacin in the topical treatment of ophthalmic infections caused by susceptible organisms.
Dosage and Administration
Administration
Moxifloxacin hydrochloride is administered orally or by IV infusion. The drug should not be given IM or by intrathecal, intraperitoneal, or subcutaneous administration.
Oral Administration
Oral moxifloxacin may be given without regard to meals. However, moxifloxacin should be administered orally at least 4 hours before or 8 hours after antacids containing magnesium, aluminum, or calcium; these same intervals also apply to concurrent therapy with moxifloxacin and other metal cations such as iron and to concurrent sucralfate, multivitamin preparations with zinc, or buffered didanosine preparations (chewable/dispersible tablets, buffered powder for oral solution, or unbuffered pediatric powder for oral solution prepared as an admixture with antacid).
IV Infusion
Moxifloxacin should be infused IV over 1 hour; rapid IV infusion of the drug should be avoided. Because only limited data are available on the physical and/or chemical compatibility of moxifloxacin and other drugs, moxifloxacin should not be admixed with other drugs or infused simultaneously through the same tubing with other drugs. Moxifloxacin IV solutions should be inspected visually for particulate matter prior to administration and should be discarded if visible particles are evident.
General Dosage
Dosage of moxifloxacin hydrochloride is expressed in terms of moxifloxacin. Dosage of oral and IV moxifloxacin is identical. When IV moxifloxacin therapy is used initially, therapy may be changed to oral moxifloxacin (when appropriate) using the same dosage to complete therapy. The timing of the change from IV to oral therapy should be individualized, taking into account the clinical status of the patient.
Respiratory Tract Infections
The usual adult dosage of moxifloxacin for the treatment of acute bacterial exacerbations of chronic bronchitis, acute sinusitis, or community-acquired pneumonia is 400 mg once daily. The usual duration of therapy is 5 days for acute bacterial exacerbations of chronic bronchitis, 10 days for acute sinusitis, and 7-14 days for community-acquired pneumonia.
Treatment of Active Tuberculosis
If oral moxifloxacin is used as an alternative agent in multiple-drug regimens for the treatment of active tuberculosis, the Centers for Disease Control and Prevention (CDC), American Thoracic Society (ATS), and Infectious Diseases Society of America (IDSA) recommend that adults and children 15 years of age or older receive 400 mg daily. These experts state that data are not available to support intermittent regimens of moxifloxacin for the treatment of tuberculosis.
Skin and Skin Structure Infection
For the treatment of uncomplicated skin and skin structure infections, the usual adult dosage of moxifloxacin is 400 mg once daily for 7 days.
Anthrax
If oral moxifloxacin is used as an alternative agent for postexposure prophylaxis following suspected or confirmed exposure to aerosolized anthrax spores (inhalational anthrax) or if oral moxifloxacin is used for the treatment of inhalational anthrax when a parenteral regimen is not available (e.g., when there are supply or logistic problems because large numbers of individuals require treatment in a mass casualty setting), the US Working Group on Civilian Biodefense suggests that adults can receive a dosage of 400 mg once daily.
Because of the possible persistence of anthrax spores in lung tissue following an aerosol exposure, the US Centers for Disease Control and Prevention (CDC) and the Working Group on Civilian Biodefense state that anti-infective therapy for treatment of inhalation anthrax or for postexposure prophylaxis should be continued for 60 days.
Special Populations
No dosage adjustment is necessary for patients with renal impairment receiving moxifloxacin. However, plasma concentrations of the sulfate and glucuronide conjugates of moxifloxacin are increased in such patients, and the clinical implications, if any, of increased exposure to these metabolites have not been studied.
The effects of hemodialysis or continuous ambulatory peritoneal dialysis (CAPD) on the pharmacokinetics of moxifloxacin have not been studied. The pharmacokinetics of moxifloxacin have been studied in a limited number of patients with mild (Child Pugh class A) and moderate (Child Pugh class B) hepatic insufficiency; the manufacturer states that no dosage adjustment is necessary for patients with mild to moderate hepatic insufficiency (Child Pugh class A and B). The pharmacokinetics of moxifloxacin have not been evaluated in patients with severe hepatic insufficiency (Child Pugh class C).
Adjustments in moxifloxacin dosage based solely on age are not necessary for geriatric patients older than 65 years of age.
Cautions
Contraindications
Known hypersensitivity to moxifloxacin, other quinolones, or any ingredient in the formulation.
Warnings/Precautions
Warnings Prolongation of QT Interval Moxifloxacin has been shown to prolong the QT interval on ECG in some patients. The manufacturer of moxifloxacin states that the drug should be avoided in patients with known prolongation of the QT interval, in those with uncorrected hypokalemia, and in those patients receiving class IA (e.g., quinidine, procainamide) or III (e.g., amiodarone, sotalol) antiarrhythmic agents because of lack of clinical experience in these patient populations.
Concomitant therapy with moxifloxacin and other drugs that prolong the QT interval (e.g., cisapride [currently commercially available under a limited-access protocol only], erythromycin, antipsychotic agents, tricyclic antidepressants) has not been studied. Since an additive effect of such concomitant therapy on QT-interval prolongation cannot be excluded, caution is advised when any of these drugs is used concurrently with moxifloxacin. In addition, moxifloxacin should be used with caution in patients with this or other ongoing proarrhythmic conditions, including clinically important bradycardia and acute myocardial ischemia.
Musculoskeletal Effects
Moxifloxacin, like most other fluoroquinolones (e.g., ciprofloxacin, gatifloxacin, levofloxacin, norfloxacin, ofloxacin), causes arthropathy in immature animals of various species.
The manufacturer states that moxifloxacin should not be used in children younger than 18 years of age and should be used during pregnancy only if the potential benefit justifies the possible risk to the fetus.
Major Toxicities Hypersensitivity Reactions
Serious and occasionally fatal anaphylactic reactions have been reported following the first dose of quinolone therapy, including moxifloxacin.
Moxifloxacin should be discontinued at the first appearance of a rash or any other sign of hypersensitivity. Clostridium difficile-associated Colitis Reported with numerous anti-infectives, including moxifloxacin; may range in severity from mild to life-threatening.
Evaluate and monitor patients who develop diarrhea during therapy.
General Precautions Nervous System Effects
Seizures, dizziness, confusion, tremors, hallucinations, depression, and suicidal thoughts or acts have been reported in patients receiving quinolones, and may occur after the first dose. If such adverse effects occur, moxifloxacin should be discontinued and appropriate measures instituted. Caution is advised in patients with known or suspected CNS disorders (e.g., severe cerebral arteriosclerosis, epilepsy) or other risk factors predisposing to seizures.
GI Effects
Nausea, vomiting, diarrhea, abdominal pain, and taste perversion.
Most of these events were mild to moderate in severity and required no treatment.
Musculoskeletal Effects
Rupture of the Achilles or other tendons requiring surgical repair or resulting in prolonged disability has been reported in patients receiving fluoroquinolones, including moxifloxacin. Postmarketing surveillance reports indicate that the risk of tendon rupture may be increased in patients receiving concomitant corticosteroids, especially geriatric patients.
Moxifloxacin should be discontinued if the patient experiences pain, inflammation, or rupture of a tendon.
Dermatologic Reactions Phototoxicity has been reported with some fluoroquinolones (e.g., ofloxacin, lomefloxacin, sparfloxacin).
While phototoxicity has not been reported to date with moxifloxacin, the manufacturer recommends avoidance of excessive sunlight or artificial UV light (e.g. tanning beds) in keeping with good medical practice.
Specific Populations Pregnancy Category C. Lactation Moxifloxacin is distributed in milk in rats; discontinue nursing or the drug because of potential for serious adverse effects in infants. Pediatric Use Safety and efficacy not established in children or adolescents younger than 18 years of age. Geriatric Use No dosage adjustment necessary. The risk of tendon rupture may be increased in geriatric patients receiving moxifloxacin concomitantly with corticosteroids.
ECG changes have been reported in geriatric patients receiving IV moxifloxacin. Renal Impairment No dosage adjustment necessary. Hepatic Impairment No dosage adjustment necessary in patients with mild to moderate hepatic insufficiency (Child Pugh class A and B). The pharmacokinetics of moxifloxacin have not been evaluated in patients with severe hepatic insufficiency (Child Pugh class C).
Common Adverse Effects
Adverse effects occurring in 3% or more of patients receiving moxifloxacin include nausea, diarrhea, and dizziness.
Drug Interactions
Drugs that Prolong QT Interval Potential pharmacologic interaction (additive effects on QT interval prolongation). (See Warnings: Prolongation of QT Interval.)
Drugs Metabolized by Hepatic Microsomal Enzymes Pharmacokinetic interaction unlikely.
Antacids
Pharmacokinetic interaction (decreased oral absorption). Moxifloxacin should be taken at least 4 hours before or 8 hours after antacids that contain aluminum, calcium, or magnesium.
Didanosine
Pharmacokinetic interaction (decreased oral absorption). If given concomitantly, buffered didanosine preparations (chewable/dispersible buffered tablets, buffered powder for oral solution, or unbuffered pediatric powder for oral solution prepared as an admixture with antacid) should be taken at least 4 hours before or 8 hours after moxifloxacin.
Digoxin, Glyburide, Probenecid, Ranitidine, and Theophylline
Pharmacokinetic interaction unlikely.
Iron, Multivitamins, Mineral Supplements, and Sucralfate
Pharmacokinetic interaction (decreased oral absorption); if given concomitantly, these drugs should be taken at least 4 hours before or 8 hours after moxifloxacin.
Itraconazole
Pharmacokinetic interaction unlikely.
Nonsteroidal Anti-inflammatory Agents (NSAIAs)
Potential pharmacologic interaction. While not observed in preclinical and clinical trials, concomitant administration of moxifloxacin and NSAIAs may increase the risk of CNS stimulation and seizures.
Warfarin
Potential pharmacologic interaction; increased prothrombin time/international normalized ratio (INR) and enhanced anticoagulant effects reported with some other quinolones (e.g., norfloxacin). While no clinically important effect of moxifloxacin on R- and S-warfarin or prothrombin time was detected in a study in healthy individuals, the manufacturer recommends careful monitoring of prothrombin time/coagulation tests in patients receiving concomitant warfarin and moxifloxacin.
Description
Moxifloxacin is a fluoroquinolone anti-infective agent. Like other commercially available fluoroquinolones, moxifloxacin contains a fluorine at the C-6 position of the quinolone nucleus. Moxifloxacin, like gatifloxacin, contains an 8-methoxy group and has been termed an 8-methoxy fluoroquinolone.
The 8-methoxy and 7-diazabicyclo moieties on the quinolone nucleus of moxifloxacin appear to enhance activity against gram-positive bacteria and decrease selection of resistant mutants in gram-positive bacteria.
Moxifloxacin is active in vitro and in clinical infections against most strains of Staphylococcus aureus (oxacillin-susceptible strains only), Streptococcus pneumoniae (including penicillin-resistant strains), S. pyogenes (group A b-hemolytic streptococci), Haemophilus influenzae, H. parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Chlamydia pneumoniae, and Mycoplasma pneumoniae.
Moxifloxacin also has in vitro activity against S. epidermidis (oxacillin-susceptible strains only), S. agalactiae (group B streptococci), viridans streptococci, Citrobacter freundii, Enterobacter cloacae, Escherichia coli, K. oxytoca, Legionella pneumophila, Proteus mirabilis, Fusobacterium species, Peptostreptococcus species, and Prevotella species; however, the safety and efficacy of moxifloxacin in treating clinical infections caused by these organisms have not been established in adequate and well-controlled clinical trials to date. In addition, moxifloxacin is active in vitro against some mycobacteria, including Mycobacterium tuberculosis, M. avium complex (MAC), M. kansasii, and M. fortuitum, and is active against some strains of M. tuberculosis resistant to isoniazid, rifampin, or streptomycin.
Moxifloxacin has greater activity in vitro against S. pneumoniae (including penicillin-resistant strains) than many other fluoroquinolones (e.g., ciprofloxacin, levofloxacin, ofloxacin) while generally retaining the in vitro activity of these drugs against gram-negative bacteria and etiologic agents of atypical pneumonia (e.g., Chlamydia pneumoniae, Mycoplasma pneumoniae, Legionella species).
The relevance of these in vitro data to the treatment of clinical infections remains to be determined. Like other fluoroquinolone anti-infectives, moxifloxacin inhibits DNA synthesis in susceptible organisms via inhibition of type II DNA topoisomerases (DNA gyrase, topoisomerase IV). In susceptible S. pneumoniae, moxifloxacin principally targets DNA gyrase. For further information on the mechanism of action of fluoroquinolones, see Mechanism of Action in Ofloxacin 8:12.. Cross-resistance can occur between moxifloxacin and other fluoroquinolones in gram-negative bacteria, but some gram-positive bacteria resistant to other fluoroquinolones may be susceptible to moxifloxacin. Moxifloxacin is well absorbed following oral administration, having an absolute bioavailability of approximately 90%.
The drug has a serum elimination half-life of about 12-15 hours), allowing once-daily dosing. Moxifloxacin is metabolized principally via sulfate and glucuronide conjugation; the drug is not metabolized by and does not appear to affect the cytochrome P-450 (CYP) enzyme system.
Advice to Patients
Importance of taking moxifloxacin at least 4 hours before or 8 hours after iron- or zinc-containing multivitamins; magnesium-, calcium-, or aluminum-containing antacids; sucralfate; or buffered didanosine preparations (chewable/dispersible buffered tablets, buffered powder for oral solution, unbuffered pediatric powder for oral solution prepared as an admixture with antacid).
Importance of informing their physician if they have history of or experience palpitations or fainting spells while on moxifloxacin. Importance of monitoring for signs of hypersensitivity reaction. Importance of informing clinicians of existing or contemplated concomitant therapy, including prescription and nonprescription drugs. Importance of reporting the persistent or worsening symptoms of infection. Importance of women informing clinicians if they are or plan to become pregnant or to breast-feed. Overview (see Users Guide).
For additional information until a more detailed monograph is developed and published, the manufacturer’s labeling should be consulted. It is essential that the manufacturer’s labeling be consulted for more detailed information on usual cautions, precautions, contraindications, potential drug interactions, laboratory test interferences, and acute toxicity.
Preparations
Moxifloxacin Hydrochloride Oral Tablets, film- 400 mg of (of moxifloxacin) Avelox®, coated Bayer Parenteral Injection, for IV 400 mg (of moxifloxacin) in Avelox® I.V. (in flexible infusion 0.8% sodium chloride containers), Bayer
Moxifloxacin: Organs and Systems
Cardiovascular
Moxifloxacin blocks the rapid-component delayed-rectifier potassium channel in the heart, and thus prolongs the QTC interval by 6 minutes after oral administration and 12 minutes after intravenous administration. Moxifloxacin carries a greater risk of QT interval prolongation than ciprofloxacin, levofloxacin, and ofloxacin, and although the risk of moxifloxacin-induced tor-sade de pointes is expected to be minimal when the drug is given in the recommended dosage (400 mg/day), moxifloxacin should be used with caution in patients with prodysrhythmic conditions and avoided in patients taking antidysrhythmic drugs, such as quinidine, procainamide, amiodarone, and sotalol.
A man developed a hypertensive crisis and transient left bundle branch block with QT interval prolongation after taking moxifloxacin.
Sinus tachycardia (120/minute) associated with moxifloxacin has been reported in a 49-year-old man about 45 minutes after he took a single 400 mg dose of moxifloxacin.
The underlying mechanism may have been vasodilatation, either directly or indirectly, owing to release of histamine, with reflex tachycardia. These effects have been described for other fluoroquinolones.
Nervous system
Syncope has been reported after the use of moxifloxacin.
Gastrointestinal
The most common adverse effects of moxifloxacin are gastrointestinal disturbances (nausea, diarrhea), which are usually mild to moderate in intensity and do not force patients to discontinue treatment. The frequency of nausea and vomiting is higher than the frequency of diarrhea and abdominal pain. Moxifloxacin caused nausea in 7.2% and diarrhea in 5.7% of patients.
In a prospective pharmacokinetic study in 12 healthy men the most frequent adverse events, possibly or probably related to moxifloxacin, were generally mild or moderate and were mostly diarrhea, nausea, and abdominal pain.
Liver
A 69-year-old man who took moxifloxacin 400 mg/day for 5 days developed cholestasis.
Skin
Warfarin
Moxifloxacin has a low propensity for causing phototoxic reactions relative to other fluoroquinolones.
Immunologic
In vitro, moxifloxacin has immunomodulatory activity through its capacity to alter the secretion of ILl-a and TNF-a by human monocytes.
Moxifloxacin can cause anaphylactic reactions.
A case of simultaneous drug allergies has been reported.
A 32-year-old woman had a generalized urticaria 15 minutes after taking co-amoxiclav and 1 year later developed a non-pruritic micropapular rash some hours after taking moxifloxacin 400 mg.
Moxifloxacin: Side Effects
Moxifloxacin is an 8-methoxyquinolone with enhanced potency against important Gram-positive pathogens, notably Streptococcus pneumoniae (penicillin-resistant and penicillin-susceptible strains), and class activity against Gram-negative bacteria. Its activity is not affected by beta-lactamases. Moxifloxacin may therefore represent a promising alternative for treatment of respiratory tract infections.
Dosage adjustment is not required for patients of advanced age or those with renal or mild hepatic impairment.
Drug interactions with moxifloxacin have been reviewed.
Observational studies
In a phase I trial, single-dose pharmacokinetics of moxifloxacin have been reported after oral administration of 50-800 mg in 45 healthy Caucasian men. Moxifloxacin was well tolerated. There were no serious adverse events, dropouts, or deaths. Only weakness was reported more often in the active treatment group. Other adverse events in subjects taking the active treatment included Herpes simplex labialis and an ear disorder. There were no changes in laboratory parameters, electrocardiograms, electroencephalograms, or findings on physical examination. Mean maximum concentrations of moxifloxacin in plasma ranged from 0.29 µg/ml (50 mg dose) to 4.73 µg/ml (800 mg dose) and were reached 0.4-4 hours after drug administration (MICs at which 90% of isolates of penicillin-resistant Streptococcus pneumoniae were inhibited were below 0.125 µg/ml). Plasma concentrations fell in a biphasic manner: within 4-5 hours, they fell to 30-55% of the Cmax. A terminal half-life of 11-14 hours accounted for most of the AUC. Protein binding was about 48%. There was partial tubular reabsorption. No major active metabolites were detected. Concentrations in saliva were higher than in the plasma during the absorption phase, whereas in the terminal phase there was a constant saliva:plasma concentration ratio of 0.5-1.
In an open, randomized, crossover study, the absolute systemic availability of a single 100 mg dose of moxifloxacin was 0.92 in 10 healthy men (mean age 29 years). There was no evidence of active tubular secretion. Both the oral and intravenous formulations were well tolerated, with five reported possible or probable drug-related adverse events, including headache, nausea, and localized urticaria.
In a prospective, uncontrolled, unblinded, phase III trial in 254 patients with community-acquired pneumonia diagnosed by culture or serologically, moxifloxacin (400 mg/day orally for 10 days) produced a bacteriological response of 91%. Drug-related adverse events were reported in 33% of patients; nausea (9%), diarrhea (6%), and dizziness (4%) were the most common adverse events.
Moxifloxacin (400 mg/day orally for 7 days) in 12 healthy men significantly reduced the normal oropharyn-geal microflora (alpha-hemolytic streptococci and Neisseriae), whereas the number of Gram-negative anero-bic bacteria increased markedly; however, no new colonizing moxifloxacin-resistant strains were isolated. Moxifloxacin caused a significant reduction in enterococci and enterobacteria, while the numbers of staphylococci, streptococci, Bacillus species, and Candida albicans were unaffected. There was no impact on peptostreptococci, lactobacilli, Veillonella, Bacteroides, or fusobacteria, but bifidobacteria and Clostridia decreased during moxifloxacin administration. The microflora normalized after 35 days.
In a multicenter, prospective, double-blind, randomized trial in 455 patients with community-acquired pneumonia oral moxifloxacin 200 mg/day or 400 mg/day for 10 days resulted in a bacteriological response of 91%. Most adverse events, possibly or probably related to moxifloxacin, were generally mild or moderate and were mostly related to the digestive system: diarrhea, nausea, and abdominal pain with 200 mg/day; diarrhea, liver function abnormalities, and nausea with 400 mg /day. The drugs were withdrawn because of adverse events in seven of 229 patients taking 200 mg/day, and 11 of 224 taking 400 mg/day.
Moxifloxacin: Organs and Systems
Drug-Drug Interactions
Antacids
The systemic availability of moxifloxacin is markedly reduced by the co-administration of antacids that contain magnesium or aluminium, unless administration occurs 2 hours before or 4 hours after moxifloxacin. An interval of 2 hours before or 4 hours after taking antacids ensures that the effect of the interaction is not clinically relevant.
Digoxin
There was no clinically relevant effect of moxifloxacin on the pharmacokinetics of digoxin in combination steady-state conditions.
Epoetin
A 76-year-old man with low-risk myelodysplastic syndrome had a major erythroid response to combination therapy with epoetin and moxifloxacin. The immunomodulatory effects of moxifloxacin may have explained the synergy with epoetin.
Iron
Iron salts impair the absorption of moxifloxacin.
Probenecid
Concomitant administration of probenecid did not affect the elimination of moxifloxacin.
Ranitidine
The systemic availability of moxifloxacin was not affected by concurrent administration of ranitidine.
Sucralfate
Drugs that contain sucralfate impair the absorption of moxifloxacin.
Theophylline
Moxifloxacin did not affect the pharmacokinetics of theophylline or vice versa.
Moxifloxacin had no effect on the pharmacokinetics of warfarin in combination steady-state conditions
Food-Drug Interactions
The effect of food on the pharmacokinetics of moxifloxacin was not clinically important. In another study dairy products had no effect on moxifloxacin kinetics.