Ceftin is a prescription drug, an antibiotic used for bacterial infection treatment. It can be prescribed as a full-prescribed therapy, as well as a part of a complex. The active ingredient in the medication is Cefuroxime axetil. It may interact with other medications and diseases, cause allergic reactions, and has quite a list of possible side effects.

However, this antibiotic is very useful for treating bacterial infections of many kinds.
In this article, you’ll learn what Cefuroxime axetil is used for, where to buy Ceftin online, what forms it has, how to store it, what Cefuroxime side effects are, etc.
What is Cefuroxime – Its Uses
Cefuroxime (Ceftin) is the 2nd generation of cephalosporin type of antibiotic. It’s used for both light symptoms and life-threatening forms of bacterial infections. It can also be prescribed by your doctor in cases not mentioned in the instruction.
The conditions treated by Ceftin are:
- Acute bacterial otitis media;
- Pharyngitis/tonsillitis;
- Acute bacterial maxillary sinusitis;
- Impetigo;
- Uncomplicated gonorrhea;
- Uncomplicated urinary tract infections;
- Bacterial infections of acute bronchitis;
- Acute bacterial exacerbations of chronic bronchitis;
- Uncomplicated skin and skin structure infections;
- Early Lyme disease.
All these conditions can be treated in adults and pediatric patients with this medicine. However, there are no clear clinical test results as to the impact on children younger than 3 months old.
It’s important to be sure that the infection was caused by susceptible bacteria if your doctor wants to prescribe the medicine. Otherwise, you may cause the development of drug-resistant bacteria.
What you should check before taking this medicine
You should discuss the usage of the medicine with your doctor and notify them about your medical history and the drugs you’re taking, starting or stopping to take, including immunizations and vaccinations. It’s also essential to know your allergies to penicillins, Cefuroxime in the form of axetil or another, etc.
Don’t share the medication with other people even if they have similar symptoms.
Allergic reaction to similar antibiotic
Do not use the medication if you’re allergic to:
- Cefdinir (Omnicef);
- Cefprozil (Cefzil);
- Cephalexin (Keflex);
- Cefaclor (Raniclor);
- Cefadroxil (Duricef);
- Cefazolin (Ancef);
- Cefditoren (Spectracef);
- Cefpodoxime (Vantin);
- Ceftibuten (Cedax);
- Cephradine (Velosef).
It’s recommended that you consult with a pharmacist if you’re not sure about the names of the medicines you’ve taken before.
List of diseases you should check before taking Ceftin
To be sure you’re safe taking Cefuroxime axetil, notify your specialist if you have any of the following or symptoms that might indicate that you have:
- Penicillin allergy;
- Liver disease (may not process the medication properly and cause a buildup);
- Kidney disease (may cause a buildup of medication in your body);
- Diabetes (the suspension form contains about 3g of sugar per teaspoon);
- Intestinal problems (Colitis, even if cured);
- Malnourishment;
- Anemia (sometimes, Cefuroxime may cause a form of anemia. If any symptoms are showing, notify the doctor immediately);
- Phenylketonuria (PKU, if the doctor prescribes the liquid form of the drug. It may contain phenylalanine);
- Any other bacterial infection (the uses of Cefuroxime may cause bacteria to overgrow, causing yeast and other infections).
It’s wise to notify your doctor about any other diseases you’ve had or allergies you’ve suffered, even if only once. Notify them if you’re pregnant, about to be pregnant, or breastfeeding. Any of these factors may alter the dosage of Cefuroxime.
Do not take the drug without a special prescription to cure conditions like the common cold or other non-bacterial infections. This may cause resistant bacteria overgrowth, meaning the medication won’t help you in the future if the need arises.
Medicine description
Cefuroxime comes in three forms:
- Cefuroxime 250mg;
- Cefuroxime 500mg;
- Cefuroxime powder for oral suspension.
Ceftin 500 mg and 250 mg tablets
The 500 mg tablets are:
- White;
- Shaped as a capsule;
- Coated with film;
- Engraves with “GXEG2” on one side;
Aside from 500 mg of Cefuroxime, they contain methylparaben, hydrogenated vegetable oil, titanium dioxide, and other ingredients.
The 250 mg tablets are:
- White;
- Shaped as a capsule;
- Coated with film;
- Engraved with “GXES7” on one side;
Aside from 250 mg of Cefuroxime, they contain methylparaben, hydrogenated vegetable oil, titanium dioxide, and other ingredients.
Ceftin for oral suspension (liquid)
The suspension comes as dry granulated powder, white or pale yellow. It has a tutti-frutti flavor. After you mix it with water, each 5 ml has 125 mg of Cefuroxime, as well as sucrose, flavoring, xanthan gum, acesulfame potassium, aspartame, stearic acid, and polyvinyl pyrrolidone.
Preparation And Administration Of Ceftin For Oral Suspension
Prepare the suspension by:
- Shaking the bottle with powder to mix and loosen it;
- Opening the bottle and add water as mentioned in the instruction;
- Closing the bottle;
- Turn the bottle upside down, rock and shake the bottle until the water gets through the powder;
- Turn the bottle upright and shake diagonally until the mixture is ready.
Make sure to shake the mixture every time before use and close the bottle tightly after each use. Store it properly in a fridge, make sure the temperature is 2 to 8C (36 and 46F). If not finished using in 10 days, dispose of the mixture and prepare a new suspension when needed.
How to administrate Ceftin tablets
The administration of Ceftin depends on the condition the medication is used for. Become familiar with the information below and read instructions to the tablets before starting the treatment.
Cefuroxime dosage for tablets
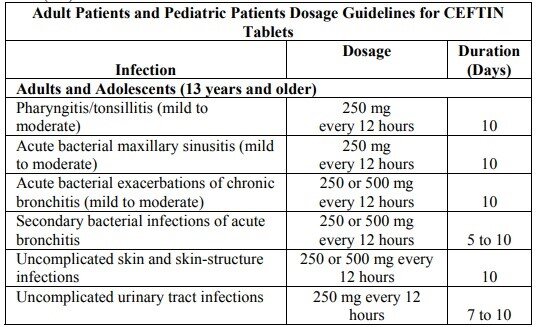 Cr: FDA
Cr: FDA
The dosages listed here are standard, but your doctor can make changes to the routine according to your condition, weight, other diseases you’ve had or are having at the moment, allergies, medications you’re using along, and other factors. Never change a dose yourself without consulting your doctor. Do not start or stop using Ceftin without a proper prescription.
Finish the whole treatment course as advised by the specialist, even if the symptoms of your bacterial infection are starting to fade away. It’s important to finish the course to cure the condition fully and avoid relapses or resistant bacteria overgrowth.
Try not to skip doses or compensate for a missed dose. If you miss the schedule, contact your doctor and get a consultation. Don’t make any decisions yourself.
Adult Patients
Adult dosage is suitable for children of 12+ years, as well as adults. The doses range from:
- 250 mg twice every day;
- to 500 mg twice every day.
Usually, the course takes a week to 10 days, but your doctor may alter the timing according to your specific case.
For the best absorption of the tablets, take them with meals. Don’t crush the tablets; swallow them whole with a whole glass of water. If it’s possible, don’t divide, chew, or crush the tablets.
Pediatric Patients
It’s recommended that pediatric patients take the suspension form of Ceftin (information on it below).
Ceftin dosage for oral suspension
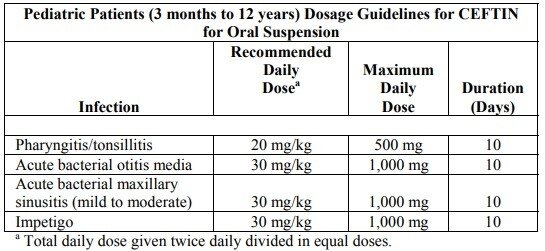 Cr: FDA
Cr: FDA
Pediatric Patients (3 Months to 12 Years)
Pediatric patients are children younger than 12 years. The dosage for such patients depends mostly on their body weight:
- 10 mg per kg two times a day;
- 15 mg per kg two times a day.
The course also lasts for a week to 10 days but may be changed by the doctor to suit your particular case. The final dosage also depends on the treated condition.
Medical syringes can be used for measuring the exact dose of medication per use. You can also use teaspoons, but if strict measurements have to be taken, the syringe is more helpful.
Missing a dose
In case you miss a dose, take one immediately after remembering. However, if it’s almost time for your next dose, avoid taking the missed one. Don’t try to compensate for a missed dose with double the amount taken the next time. This may cause an overdose.
Ceftin overdose
If you confuse the scheduling and accidentally overdose on Ceftin, call 911 immediately or reach the Poison Helpline at 1-800-222-1222. If you feel like you’re about to have a seizure, reach out to people around.
Overdose can be accompanied by seizures, convulsions, or black-outs.
Cefuroxime side effects
Cefuroxime has a list of side effects. Some of them require immediate medical attention, while others may wait until your next appointment. Here are the most common side effects of Ceftin:
- Diarrhea;
- Nausea;
- Vomiting;
- Skin rash from a diaper in infants;
- Change in taste.
It’s still important to tell your doctor if you have any of these side effects. If they don’t go away, you may need a change in the dose.
Also, keep in mind that the effects presented in medical instructions are not a full list. There may be other individual effects you’ll have to discuss with your doctor. If you encounter any unusual issues connected to the usage of Cefin, you can also report them to FDA at 1-800-FDA-1088.
Requiring immediate medical attention
Side effects requiring immediate medical help are:
- Allergic reactions like difficulty breathing, swelling, hives;
- Severe stomach pain;
- Watery or bloody diarrhea;
- Skin rash;
- Easy bruising;
- Numbness;
- Strong tingling;
- Yellowing of your eyes and/or skin;
- Seizure;
- Issues with kidney work (problems with urination, severe swelling in your feet, fatigue, shortness of breath);
- High fever;
- Burning in the eyes;
- Skin pain, blistering, peeling.
If you notice any of the symptoms that are very painful or making you very uncomfortable, seek medical help as soon as possible. Have your doctor’s number nearby to contact them right away, along with the ambulance.
Pregnancy or Breastfeeding Warnings
Tell your doctor if you’re pregnant, plan to be pregnant in the near future, or are breastfeeding. While there are no proven cases of harmful impact on an unborn baby, the doctor might need to alter the dose or schedule of your treatment.
Cefuroxime is passed into breast milk and may cause allergic and other reactions in your baby.
Keep in mind that Cefuroxime makes hormonal birth control less effective. Consult your doctor on whether you should try other means of contraception.
It’s not recommended to use Cefuroxime for children who are younger than 3 months old unless absolutely necessary.
What will affect Ceftin – Interactions
Cefuroxime interactions are plenty, that’s why you must tell your doctor about your diet, medications you’re taking, medical conditions, etc. More on that below.
Alcohol/food interactions
It’s highly recommended to take tablets of Ceftin with meals. The bioavailability increases by almost 20% when you take a dose with food. However, the bacteriologic response is the same, not depending on how you take the tablets, with food or without it. There were 2 tests on the topic.
There’s no sufficient information on the absorption improvement after taking an oral suspension with or without an accompanying meal.
As to Cefuroxime axetil interaction with alcohol, there are no strict warnings as to whether alcohol intake can disrupt the absorption, excretion, or efficacy of Cefuroxime. However, if you experience side effects connected to digestion or headache, alcohol intoxication may make things worse.
Disease interactions
The medicine will interact with the following diseases:
- Colitis;
- Renal dysfunction;
- Liver disease;
- Seizure disorders;
- Ferricyanide tests;
You should notify your doctor about any of these conditions before starting the treatment. Any of these may cause product buildup in your blood, swelling, severe side effects.
Medicines interactions
Cefuroxime medicine interactions include:
- Aminoglycoside antibiotics (amikacin, gentamicin, tobramycin, etc.);
- H2 antagonists (famotidine, ranitidine, etc.);
- Antacids (aluminum hydroxide, calcium carbonate, magnesium hydroxide, etc.);
- Hormonal birth control;
- BCG;
- Disulfiram;
- Sodium picosulfate;
It also interacts with the following vaccines:
- BCG vaccine;
- Cholera vaccine;
- Typhoid vaccine.
Pay attention to all of your prescriptions and see if you have any of the above taken right before, within, or right after the course of Ceftin. Notify your doctor if you have to take any of those medicines. In your particular care, this might mean you’ll have to either stop taking/substitute any of the medications in the course or change the doses of one or more of them.
Keep in mind that interaction between medicines doesn’t mean that you will have to stop taking any of them. There are cases when everything remains as usual, including the schedule and dosage of every drug. The decision depends on your condition, its severity, any side effects, or other factors determined by your doctor.
The medications mentioned above aren’t the only ones that may interact with Ceftin; there are others. So, if you take any other medicines, make sure your specialist knows about any of them. And if you notice any slight change in your condition, notify the doctor immediately. Sometimes the interactions depend on the condition of your organism.
Also, if you’re a frequent alcohol or coffee drinker or cigarette smoker, it’s worth mentioning at your appointment.
Any blood-thinners, anti-diarrhea pills, etc. may interact with antibiotics as well, so don’t take them without consulting a professional.
Storage And Handling
Proper storage and handling of any drug are imperative for its efficacy. Here are some things you must know about storing, handling, and disposing of Ceftin:
- Read the label and medical instruction carefully and follow every direction;
- Don’t take the medicine in a larger or smaller amount than prescribed;
- To avoid unpleasant feelings, don’t crush or chew the tablet;
- Shake the liquid well before measuring a dose;
- Make sure it’s properly refrigerated (2-8C);
- Dispose of it if not used for 10 days;
- If changing the form of the medicine mid-treatment, consult on proper measurements;
- Don’t stop the treatment unless your specialist says so, even if the symptoms seem to fade away;
- Don’t try to treat common non-bacterial infections with Ceftin; visit a doctor for a proper prescription;
- If you’re about to have urine tested, keep in mind that the sugar levels in it might be higher than usual;
- Store the tablets at room temperature;
- Choose a dry, cool place for storage;
- Make sure the cap is closed tightly when the tablets aren’t used;
- Store the suspension in a fridge but never allow it to freeze; it will interfere with the integrity of the medicine and lower its efficacy;
- Keep Ceftin in any form out of reach of your children or pets;
- Never dispose of any medicine, including Ceftin, in wastewater or simply throwing it away with your usual trash.
For more recommendations on the disposal of any particular medicine, contact your doctor or visit a local pharmacy. Some cities have special centers for proper disposal of medications. If you don’t have one, consider mixing the tablets or suspension with cat litter, dirt, coffee grounds, etc. Use something that a person or an animal won’t try to dig in and eat the tablets. Put the resulting mixture in a zip bag or another closed container and throw it away with other household trash.
| Dosage forms of Cefuroxime: | |||
|---|---|---|---|
| Apo-Cefuroxime 250 mg Tablet | Ratio-Cefuroxime 250 mg Tablet | Velosef 250 mg capsule | Ceftin 250 mg Tablet |
| Velosef 500 mg capsule | Apo-Cefuroxime 500 mg Tablet | Ratio-Cefuroxime 500 mg Tablet | Cefuroxime axetil 250 mg tablet |
| Ceftin 500 mg Tablet | Cefzil 250 mg tablet | Duricef 1 gm tablet | Cefuroxime axetil 500 mg tablet |
| Cefzil 500 mg tablet | Ceftin 250 mg tablet | Cefuroxime sod 1.5 gm vial | Zinacef 1.5 gm vial |
| Zinacef 1.5 gm add-vant vial | Cedax 400 mg capsule | Cefuroxime 1.5 g/50 ml bag | Ceftin 500 mg tablet |
| Maxipime 1 gram vial | Duricef 250 mg/5ml Suspension 50ml Bottle | Maxipime 1 gm piggyback vial | Velosef 250 mg/5ml Suspension 100ml Bottle |
| Maxipime 2 gram vial | Duricef 500 mg/5ml Suspension 75ml Bottle | Cefzil 125 mg/5ml Suspension 100ml Bottle | Duricef 500 mg/5ml Suspension 100ml Bottle |
| Rocephin 1 gm vial | Rocephin 1 gm Solution Vial | Zinacef 7.5 gm vial | Cefzil 250 mg/5ml Suspension 100ml Bottle |
| Rocephin 2 gm vial | Cefuroxime Axetil 250 mg/5ml Suspension 100ml Bottle | Ceftin 20 500 mg tablet Bottle | Rocephin 10 gm vial |
| Zinacef-water 1.5 gm/50 ml | |||
Synonyms of Cefuroxime:
Cefuroxim, Cefuroxime [USAN:BAN:INN], Cefuroximo [INN-Spanish], Cefuroximum [INN-Latin]
How can i get Cefuroxime online over the counter?
You can buy Cefuroxime OTC in online drugstore with low cost.
Therapeutic classes of Cefuroxime:
Anti-Bacterial Agents, Cephalosporins
Delivery
Australia, Canada, Mexico, New Zealand, USA, Europe [Belgium, France, Norway, Holland, Ireland, Spain, Switzerland, Great Britain (UK), Italy] and etc.