Buy Bactrim (Co-trimoxazole)
Bactrim, also known as co-trimoxazole, is an antibiotic used to treat various bacterial infections. It is available in both tablet and liquid forms. The name “Co-trimoxazole” reflects its dual active ingredients, which work together to combat bacterial infections.
Bactrim: Known by What Other Name?
This medication is marketed under various names in different countries. In the United States, it is commonly referred to as Bactrim and Septra, while in Canada, it may be found as Apo-Sulfatrim or Novo-Trimel. Other names include Sulfatrim and SMZ-TMP in pediatric formulations.
In Europe, Bactrim is primarily marketed under the brand name Bactrimel, which is manufactured by Roche. Other names include Cotrim and Septra, which also combine sulfamethoxazole and trimethoprim. Additionally, variations like Biseptol and Sumetrolim may be found in certain countries. These brand names can vary by country.
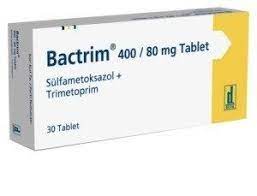 What Is Bactrim?
What Is Bactrim?
Bactrim (Co-trimoxazole) is a combination antibiotic that contains sulfamethoxazole and trimethoprim. It is primarily used to treat various bacterial infections, such as urinary tract infections, ear infections, bronchitis, and pneumonia. The medicine works by inhibiting the production of folic acid in bacteria, which is essential for their growth and survival. This medication is effective against a wide range of bacterial pathogens.
Composition and Active Ingredients
Bactrim is a fixed-dose combination antibiotic that contains two active ingredients:
- sulfamethoxazole, a sulfonamide antibiotic that inhibits bacterial growth by interfering with the production of folic acid, an essential nutrient for bacteria;
- trimethoprim is an antifolate antibiotic that works by blocking the enzyme dihydrofolate reductase, which is crucial for synthesizing nucleic acids in bacteria.
Combining these two antibiotics enhances their effectiveness against a broad spectrum of bacterial pathogens. These two active ingredients make Bactrim a versatile treatment option for various infections. The typical ratio in this medicine formulation is one part trimethoprim to five parts sulfamethoxazole, which optimizes their synergistic effects.
Bactrim is rapidly absorbed following oral administration. Both sulfamethoxazole and trimethoprim exist in the blood as unbound, protein-bound, and metabolized forms.
Peak blood levels for the individual components occur 1 to 4 hours after oral administration. The mean serum half-lives of sulfamethoxazole and trimethoprim are 10 and 8 to 10 hours, respectively. However, patients with severely impaired renal function exhibit an increase in the half-lives of both components, requiring dosage regimen adjustment.
The kidneys primarily secrete sulfamethoxazole and trimethoprim through both glomerular filtration and tubular secretion.
Use Cases
Bactrim is indicated for the treatment of numerous bacterial infections, such as:
- urinary tract infections (UTIs) – effective against common pathogens such as Escherichia coli and Klebsiella;
- acute otitis media – in pediatric patients, when other treatments may be ineffective;
- bronchitis – used for acute exacerbations caused by susceptible strains;
- traveler’s diarrhea – effective against enterotoxigenic E. coli;
- Pneumocystis jirovecii pneumonia (PCP) – used as a preventive measure in immunocompromised individuals, such as those with HIV/AIDS.
Additionally, Bactrim can prevent infections like toxoplasmosis and nocardiosis in specific high-risk populations.
Do I Need a Prescription?
Yes, Bactrim is classified as a prescription medication. Contact your healthcare provider. They can evaluate the necessity of the treatment based on the specific infection and patient history. Self-medication with antibiotics can lead to ineffective treatment and contribute to antibiotic resistance.
Co-trimoxazole Dosing and Warnings
Bactrim is supplied in various forms, primarily as oral tablets and liquid formulations. The most common tablet strengths include Bactrim 400 mg of sulfamethoxazole combined with 80 mg of trimethoprim. There is also a double-strength version, Bactrim DS, which contains 800 mg of sulfamethoxazole and 160 mg of trimethoprim.
Additionally, Bactrim is available in a liquid form for pediatric use. The dose is adjusted based on the child’s weight. These formulations allow for flexibility in dosing depending on the patient’s specific needs and the type of infection being treated.
| Dosage Form | Strength | Brand Name | Composition/Features |
|---|---|---|---|
| Tablets | 400 mg Sulfamethoxazole / 80 mg Trimethoprim | Bactrim | Standard formulation |
| Tablets, DS (Double Strength) | 800 mg Sulfamethoxazole / 160 mg Trimethoprim | Bactrim DS | Double strength |
| Oral Suspension | 200 mg Sulfamethoxazole / 40 mg Trimethoprim per 5 mL | Bactrim Suspension | Sweetened for pediatric use |
| Intravenous (IV) Solution | 80 mg Trimethoprim / 400 mg Sulfamethoxazole per 5 mL | Bactrim IV | For hospital use |
How and When to Take
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Bactrim (sulfamethoxazole and trimethoprim) tablets and other antibacterial drugs, the medicine should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. Treatment with Bactrim (sulfamethoxazole and trimethoprim) tablets without a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to benefit the patient. It increases the risk of the development of drug-resistant bacteria.
Bactrim is typically administered twice daily, with or without food. The specific dosage depends on the type of infection being treated:
| Condition | Patient Group | Dosage |
|---|---|---|
| Urinary Tract Infections | Adults | Bactrim DS (800 mg sulfamethoxazole/160 mg trimethoprim) one tablet every 12 hours for 10 to 14 days. |
| Children (2 months and older) | Dosage based on weight: approximately 40 mg/kg sulfamethoxazole and 8 mg/kg trimethoprim daily, divided into two doses. | |
| Acute Otitis Media | Children | Similar weight-based dosing as for urinary tract infections. |
| Bronchitis | Adults | One Bactrim DS tablet every 12 hours for 14 days. |
Taking Bactrim at evenly spaced intervals is essential to maintain adequate drug levels in the bloodstream.
In Case You Forget to Take It
If you miss a dose of Bactrim, take it as soon as you remember. If it’s close to the time for your next dose, skip the missed dose—do not double up. Consistency in taking your medication helps maintain its effectiveness.
What to Do in Case of Overdose
In case of an overdose, seek immediate medical attention or contact poison control. Symptoms may include nausea, vomiting, dizziness, or confusion. Prompt intervention is essential to manage potential complications from an overdose.
Precautions
Several precautions should be observed when taking Bactrim to ensure safety and efficacy. As dosage adjustments may be necessary, inform your healthcare provider about any pre-existing medical conditions, particularly kidney or liver issues.
Additionally, Bactrim is not recommended for pregnant or breastfeeding women due to potential risks to the unborn child or nursing infant. Patients should also be cautious of possible allergic reactions. They should discontinue use and seek medical attention if severe skin reactions or other serious side effects occur.
Patients should be instructed to maintain an adequate fluid intake to prevent crystalluria and stone formation.
When Bactrim (sulfamethoxazole and trimethoprim) tablets are indicated to treat a bacterial infection, patients should be told that although it is expected to feel better early in therapy, the medication should be taken exactly as directed.
Allergy Warnings
Patients with a known allergy to sulfonamide antibiotics should avoid taking Bactrim due to the risk of severe allergic reactions, including Stevens-Johnson syndrome, anaphylaxis, toxic epidermal necrolysis, allergic myocarditis, exfoliative dermatitis, erythema multiforme, angioedema, drug fever, HenochSchoenlein purpura, chills, serum sickness-like syndrome, generalized skin eruptions, generalized allergic reactions, photosensitivity, conjunctival and scleral injection, pruritus, urticaria and rash. Also periarteritis nodosa and systemic lupus erythematosus have been reported. It is crucial to inform healthcare providers about any previous allergic reactions before treatment.
Health Conditions Warnings
Individuals with certain health conditions should use Bactrim cautiously:
- patients with impaired renal function may require dosage adjustments due to reduced clearance of the drug;
- caution is advised for patients with liver impairment, as this condition can affect drug metabolism;
- patients with conditions like porphyria or megaloblastic anemia should avoid this medication unless specifically directed by their physician.
This medicine is contraindicated in pediatric patients less than 2 months of age.
Fatalities associated with the administration of sulfonamides, although rare, have occurred due to severe reactions, including Stevens-Johnson syndrome, fulminant hepatic necrosis, aplastic anemia, toxic epidermal necrolysis, agranulocytosis, and other blood dyscrasias.
AIDS patients may not tolerate or respond to Bactrim in the same manner as non-AIDS patients.
Regular monitoring may be necessary during treatment for those with pre-existing conditions.
Co-trimoxazole Side Effects
Common side effects associated with Bactrim include:
- nausea and vomiting;
- rash or itching;
- diarrhea.
Serious side effects can occur but are rare; these include:
- severe allergic reactions (e.g., Stevens-Johnson syndrome, anaphylaxis, toxic epidermal necrolysis, allergic myocarditis, exfoliative dermatitis, erythema multiforme, angioedema, drug fever, HenochSchoenlein purpura, chills, serum sickness-like syndrome, generalized skin eruptions, generalized allergic reactions, photosensitivity, conjunctival and scleral injection, pruritus, urticaria and rash, also periarteritis nodosa and systemic lupus erythematosus have been reported.);
- blood disorders such as thrombocytopenia or leukopenia;
- gastrointestinal: hepatitis (including cholestatic jaundice and hepatic necrosis), elevation of serum transaminase and bilirubin, pseudomembranous enterocolitis, stomatitis, pancreatitis, glossitis, nausea, abdominal pain, emesis, diarrhea, anorexia;
- neurologic: convulsions, aseptic meningitis, peripheral neuritis, vertigo, ataxia, tinnitus, headache.
- psychiatric: depression, nervousness, hallucinations, apathy.
- endocrine: the sulfonamides bear certain chemical similarities to some goitrogens, diuretics (acetazolamide and the thiazides), and oral hypoglycemic agents; cross-sensitivity may exist with these agents. Diuresis and hypoglycemia have occurred rarely in patients receiving sulfonamides;
- musculoskeletal: arthralgia and myalgia; isolated cases of rhabdomyolysis have been reported with Bactrim, mainly in AIDS patients;
- respiratory: cough, shortness of breath, and pulmonary infiltrates;
- miscellaneous: fatigue, weakness, insomnia.
Clinical signs, such as rash, sore throat, fever, arthralgia, pallor, purpura, or jaundice, may indicate severe reactions.
Patients should promptly report any unusual symptoms or side effects to their healthcare provider.
Interaction with Other Medicines
Bactrim can interact with several medications. This may increase the risk of adverse effects or reduce therapeutic efficacy. The interactions include:
- Warfarin (increased risk of bleeding due to enhanced anticoagulant effect);
- Methotrexate (potentially increased toxicity due to similar mechanisms affecting folate metabolism);
- Diuretics (certain diuretics may increase potassium levels when taken with Bactrim).
Always inform your healthcare provider about all medications you are currently taking, including over-the-counter drugs and supplements, to avoid potential interactions.
After Using Bactrim Medication
After using Bactrim medication, it is essential to store it properly. Unused or expired pills should also be disposed of properly.
Storage
Bactrim should be stored at room temperature, away from moisture and heat sources. Keep it out of reach of children and pets. Proper storage helps maintain the medication’s efficacy throughout its shelf life.
Disposal
Do not flush unused or expired pills down the toilet unless instructed. Consult your pharmacist for safe disposal methods or local guidelines on medication disposal.
Bactrim Tablet Alternatives
Several alternatives are available for those who cannot take Bactrim due to allergies or other contraindications. These alternatives include:
- Nitrofurantoin, often used specifically for urinary tract infections;
- Ciprofloxacin, a fluoroquinolone antibiotic effective against various bacterial infections;
- Amoxicillin, a penicillin-type antibiotic used for different types of infections.
Consult your healthcare provider for recommendations based on your specific condition and medical history.
Bactrim (Co-trimoxazole) is a powerful combination antibiotic that effectively treats many bacterial infections. Understanding its composition, uses, precautions, dosing guidelines, potential side effects, and interactions with other medications is crucial for safe and effective use. Always follow your healthcare provider’s instructions regarding its use and report any adverse effects or concerns during treatment.


 (2 votes, average: 4.50 out of 5)
(2 votes, average: 4.50 out of 5)