If you are looking for a reliable online pharmacy store, you can place an order at our sponsor and support drug guide. You will find the best generic medicines at low prices online. Buy high-quality Flagyl (Metronidazole) online. Quick worldwide delivery and exclusive hot discounts are provided to every customer.
The name of this medicine is Flagyl (Metronidazole). This belongs to a group of medicines called antibiotics. It works by killing bacteria and parasites that cause infections in your body.
What Is Metronidazole?
It can be used to:
- treat infections of the blood, brain, lungs, bones, genital tract, pelvic area, stomach, and intestines;
- treat gum ulcers and other dental infections;
- treat infected leg ulcers and pressure sores.
Metronidazole is effective in the treatment of many protozoal diseases, notably trichomoniasis, amebiasis, schistosomiasis, strongyloidiasis, and giardiasis, and has been in use for over 20 years.
For amebiasis there is still discussion about the use of a single high dose versus repeated lower doses, both as regards efficacy and adverse effects. The use of metronidazole against infections with anerobic bacteria has increased over the years, and with this indication the use of metronidazole in combination with many other drugs used by patients with conditions likely to develop secondary anaerobic bacterial infections. With increased use there is also a widespread and increasing incidence of resistance of various strains of bacteria. The use of metronidazole as an added medication merely “to make assurance double sure” is to be discouraged. It is to be especially discouraged in immunocompromised patients, because of the risk of emergence of resistant bacterial strains. With increased use, there is also an increased number of reports of some more unusual adverse effects. Overall, metronidazole can still be considered safe if used in generally recommended doses.
Metronidazole has been formulated as a vaginal gel (0.75%) for the treatment of bacterial vaginosis. A single daily 5-day regimen has been approved by the FDA and has been shown to be as effective as oral metronidazole. In the eradication of Helicobacter pylori, metronidazole plus bismuth is effective but causes more adverse effects than omeprazole plus amoxicillin plus either clarithromycin or metronidazole.
Pharmacokinetics
Metronidazole has excellent systemic availability, and absorption after oral administration is not significantly affected by food. Peak serum concentrations occur about 1 hour after ingestion. Multiple doses every 6-8 hours result in some drug accumulation, and the half-life averages about 8 hours. There is a linear relationship between dose and serum concentration. Rectal administration results in serum concentrations about half those seen after oral administration.

Systemic absorption after local use in the vagina is slow, with maximum serum concentrations being reached only after 8-24 hours; they are only about 20% of those attained after oral administration.
Metronidazole is extensively metabolized, and only about 20% of the dose is excreted unchanged in the urine. Tissue concentrations are similar to serum concentrations, and metronidazole penetrates readily into the central nervous system. As with many metabolized drugs, the half-life is markedly prolonged in neonates; there is an inverse relation between gestational age and half-life.
Prolonged elimination times are also seen in the presence of serious liver disease. Preliminary case reports have suggested that azithromycin or metronidazole can improve ciclosporin-induced gingival hyperplasia.
How to Take Metronidazole
Always take the medicine exactly as your doctor has told you. It is important to finish a full course of treatment. The length of a course will depend on your needs and the illness being treated.
Check with your doctor or pharmacist if you are not sure.
- Swallow the tablets whole with a drink of water
- Do not crush or chew the tablets
- Take these tablets during or just after a meal
- The dose will depend on your needs and the illness being treated
- The length of your treatment will depend on the type of illness you have and how bad it is
The usual dose for adults and children is given below:
| Indication | Patient Group | Dosage | Frequency |
|---|---|---|---|
| To treat bacterial infection | Adults | Initial: 800 mg | Every 8 hours |
| Subsequent: 400 mg | |||
| Children | Dose based on weight (7.5 mg/kg) | Every 8 hours | |
| To prevent infections after surgery | Adults | Start 24 hours before surgery: 400 mg | Every 8 hours |
| Post-operative: may be given via drip or rectally until able to take tablets | |||
| Children | Dose based on weight | Every 8 hours | |
| Start 24 hours before surgery | |||
| Post-operative: may be given via drip or rectally until able to take tablets |
Your doctor will decide how much and how often to take, depending on the severity of your illness, to treat other infections caused by parasites and some bacteria.
People with Kidney Dialysis
Kidney dialysis removes Metronidazole from your blood. If you are having kidney dialysis, you must take this medicine after your dialysis treatment.
People with Liver Problems
Your doctor may tell you to use a lower dose or to use the medicine less often.
If You Take More Metronidazole Than You Should
If you take more than you should, contact your doctor or the nearest hospital emergency department immediately. If possible, always take the box, this leaflet, and any tablets that are left over with you so the doctor knows what you have taken.
If You Forget to Take the Dose
If you miss a dose, take it as soon as you remember and continue as usual. If it is almost time for your next dose, skip the forgotten dose and continue as usual. Do not take a double dose to make up for a missed dose.
If you have any further questions about the use of this product, ask your doctor or pharmacist.
Important Safety Information
Do not take Metronidazole Tablets if you are allergic (hypersensitive) to Metronidazole or any of the other ingredients in your medicine. Signs of an allergic reaction include a rash, swallowing or breathing problems, and swelling of your lips, face, throat, or tongue.
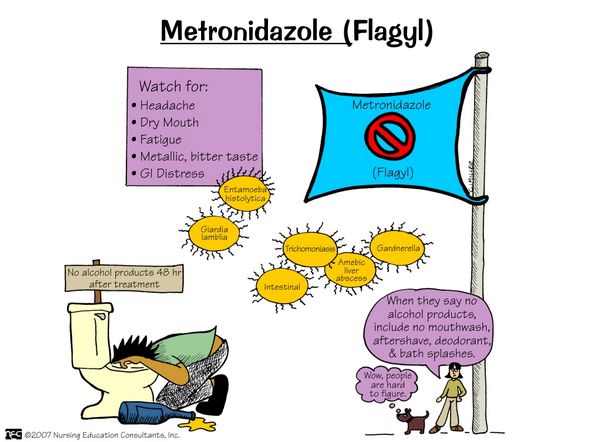
Do not drink alcohol while you are taking Metronidazole and for 48 hours after finishing your course. Drinking alcohol while using Metronidazole might cause unpleasant side effects, such as feeling sick (nausea), being sick (vomiting), stomach pain, hot flushes, very fast or uneven heartbeat (palpitations), and headache.
Pregnancy and Breastfeeding
Tell your doctor before using this medicine if:
- you are pregnant, might become pregnant, or think you may be pregnant. Metronidazole should not be taken during pregnancy unless considered absolutely necessary;
- you are breastfeeding. It is better not to use Metronidazole if you are breastfeeding. This is because small amounts may pass into the mother’s milk.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and Using Machines
While taking Metronidazole, you may feel sleepy, dizzy, confused, see or hear things that are not there (hallucinations), have fits (convulsions), or temporary eyesight problems (such as blurred or double vision). If this happens, do not drive or use any machinery or tools.
Your doctor may wish to carry out some tests if you have been using this medicine for more than 10 days.
Possible Side Effects
Metronidazole is generally well tolerated. With high doses and high-dose prolonged treatment, nausea, vomiting, and central nervous system symptoms ranging from headache and dizziness to neuritis can occur. The commonest reactions are nausea, a metallic taste in the mouth, furry tongue, and vulvovaginal irritation in patients who take metronidazole for bacterial vaginosis. Reports of pancreatitis, neuropathy, and optic neuritis call for caution. Hypersensitivity reactions are unusual, but rashes have been described.
Stop taking Metronidazole and see a doctor or go to a hospital straight away if you get swelling of the hands, feet, ankles, face, lips or throat which may cause difficulty in swallowing or breathing. You could also notice an itchy, lumpy rash (hives) or nettle rash (urticaria). This may mean you are having an allergic reaction.
A serious but very rare side effect is a brain disease (encephalopathy). The symptoms vary, but you might get a fever, stiff neck, headache, or or see or hear things that aren’t there. You might also have problems using your arms and legs, problems with speaking, or feel confused.
Talk to your doctor straight away if you notice the following side effects.
- Yellowing of the skin and eyes. This could be due to a liver problem (jaundice).
- Unexpected infections, mouth ulcers, bruising, bleeding gums, or severe tiredness. This could be caused by a blood problem.
- Severe stomach pain which may reach through to your back (pancreatitis).
Tell your doctor or pharmacist if you notice any of the following side effects:
Very Rare (affects less than 1 in 10,000 people)
- Fits (convulsions).
- Mental problems such as feeling confused and seeing or hearing things tha.t are not there (hallucinations).
- Problems with your eyesight, such as blurred or double vision.
- Skin rash.
- Headache.
- Darkening of the urine.
- Feeling sleepy or dizzy.
- Pains in the muscles or joints.
Side Effects with Unknown Frequency
- Numbness, tingling, pain, or a feeling of weakness, in the arms or legs.
- Unpleasant taste in the mouth.
- Furred tongue.
- Feeling sick (nausea), being sick (vomiting), upset stomach, or diarrhoea.
- Loss of appetite.
If any of the side effects becomes serious or last longer than a few days,, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Long-Term Effects
Drug Tolerance
Resistance of H. pylori to metronidazole was found in 30% of isolates in Lebanon, 42% in Brazil, and 80-90% in Africa.
Mutagenicity
Mutagenicity of metronidazole has been demonstrated in some bacterial systems. Studies on breakages in single-stranded DNA in the lymphocytes of patients treated with metronidazole for Trichomonas vaginitis have suggested that such breakages were repaired after withdrawal.
Another study reported chromosomal aberrations in the lymphocytes of ten volunteers taking metronidazole. A mutagenic effect would theoretically be possible in patients with a DNA repair defect. Concern that metronidazole may be genotoxic has been raised, as several bacterial species have been reported to be mutagenic.
The genotoxic effects of metronidazole (250 mg bd for 10 days) and nalidixic acid (400 mg bd for 10 days) have been assessed in women with Trichomonas vaginalis infections. The genotoxic potential of these drugs was evaluated using a sister chromatid exchange test in peripheral blood lymphocytes. Metronidazole had no effect, but nalidixic acid increased the frequency of sister chromatid exchange. This result confirms that there is little evidence of genotoxicity with metronidazole.
Tumorigenicity
Prolonged high-dose exposure of mice to metronidazole leads to an increased incidence of lung tumors, and in one study, there was an increase in lymphoreticular neoplasia in female animals. These results, which caused much concern when first published, are probably non-specific and not relevant to humans; these and other neoplasms have also been induced in mice merely by varying the diet.
Several long-term follow-up studies in men have failed to demonstrate an excess cancer risk. There has been a single report of cancers in three patients with Crohn’s disease who had taken metronidazole for years, but they had also taken sulfasalazine and glucocorticoids, and this cannot be regarded as constituting reasonable evidence of a causal link.
Second-Generation Effects Teratogenicity
Tests for embryotoxicity and teratogenicity in different animal species have been negative, and there have been no reports of adverse effects on the fetus in pregnant women given metronidazole for trichomoniasis. Despite this, it is still wise to avoid metronidazole during the first trimester of pregnancy. In a retrospective cohort study using the national birth registry in Denmark, comparing 124 women who took the drug with 13 327 who did not, there was no evidence of any increased risk to the unborn child.
In a prospective case-control study in Israel of 857 pregnant women seeking telephone advice regarding gestational exposure to prescribed drugs, 228 women who had taken metronidazole were compared with 629 controls exposed to non-teratogenic agents.
The mean daily dose of metronidazole was 973 mg, lasting 7.9 days. Ninety percent had used the medication orally, 6% by suppository, and 4% intravenously. Most (86%) had been exposed to metronidazole in the first trimester of pregnancy. The rate of major congenital malformations between the groups was the same (1.6 versus 1.4%), even after accounting for terminations due to prenatally diagnosed malformations.
Neonatal birth weight was reduced in the metronidazole group (3.2 versus 3.3 kg), and this was not explained by an earlier gestational age at delivery or a higher prematurity rate but may have been due to the underlying conditions for which metronidazole was prescribed. These findings agree with previous meta-analyses showing that the use of metronidazole in pregnancy is not associated with an increased risk of fetal abnormality, despite in vitro evidence of mutagenesis and inconsistent animal evidence of fetal abnormalities caused by metronidazole.
Lactation
Metronidazole is excreted in the breast milk. There were no adverse effects in nursing infants, but one should still be cautious in using metronidazole in nursing mothers.
Age
In a randomized trial in 100 Iranian children, mebendazole (200 mg tds for 5 days, n — was compared with metronidazole (5 mg/kg tds for 7 days, in giardiasis. The two drugs were equally effective (over 85% cure rates). There were no adverse effects of mebendazole, whereas nausea, anorexia, and metallic taste were respectively observed in 4.9, 6, and 24% of those taking metronidazole.
Metronidazole’s Effect on Organs and Systems
Cardiovascular
Thrombophlebitis can occur after intravenous administration of metronidazole; an incidence of 6% has been cited.
Respiratory
Pneumonitis has been attributed to metronidazole, with recurrence after rechallenge.
Nervous system
Central nervous system symptoms can occur with standard doses of metronidazole, but they are mainly seen with high doses, especially when such doses are given for a long time. Under the latter conditions, there was a 25% incidence of such symptoms as headaches, dizziness, tremors, ataxia, and confusion. A polyneuropathy is a well-recognized adverse effect of metronidazole. A 6% incidence of neuropathy has been quoted; polyneuropathy, mainly sensory in nature, has been recorded during the treatment of Crohn’s disease, but again in connection with the prolonged use of high doses.
This complication is not restricted to patients with Crohn’s disease; it is also seen when metronidazole is given for other purposes, such as radiosensitization. Electrophysiological studies have suggested distal sensory axonal degeneration, with loss of sensory nerve potential over the distal segment and normal motor nerve conduction. In some cases, the severity of clinical and electrophysiological abnormalities is closely related to the total amount of metronidazole administered. Metronidazole is structurally similar to thiamine, and thiamine-synthesizing gut flora may synthesize a neurotoxic analog of thiamine from ingested metronidazole. However, this hypothesis is weakened by the fact that nitro heterocyclic drugs other than metronidazole are also neurotoxic, despite a much weaker structural analogy to thiamine. Peripheral neuropathy was associated in one case with intermittent use of metronidazole (2 g/day for 5 days every other month).
A 65-year-old white woman with small intestine bacterial overgrowth developed persistent numbness and tingling in her upper and lower extremities. She had been taking alternating courses of tetracycline and metronidazole for 5 days every other month for about 1 year. Other medications included amitriptyline, lisinopril, digoxin, omeprazole, and tamoxifen. Serum vitamin Bi2 and folate concentrations were within the reference ranges. She had reduced sensation in a stocking-glove distribution, reduced sensation to touch and pin-prick, intact reflexes, and no weakness. Neuropathy was attributed to metronidazole, which was withdrawn.
After 4 months, she reported improvement. On follow-up at 5 months, there was no evidence of peripheral neuropathy. Metronidazole-associated sensory neuropathy usually presents with pain and reduced thermal and pin-prick sensation but normal strength, proprioception, and tendon reflexes. Four patients with metronidazole-associated sensory symptoms had detailed electrodiagnostic studies and nerve or muscle biopsies. All had taken different doses of metronidazole, and one (with a mitochondrial myopathy) had only used the drug topically. After withdrawal, sensory complaints persisted without progression in two patients and resolved in one. In the fourth, a lower dosage led to partial resolution.
Nerve conduction studies were normal in all four cases, but quantitative sensory testing and quantitative sudomotor autonomic reflex testing showed abnormalities of small-fiber function. A sural nerve biopsy from one patient confirmed some loss of small myelinated axons.
Metronidazole probably caused small-fiber neuropathy in these four cases. The authors also reviewed nerve conduction studies in 30 reported cases of metronidazole-associated neuropathy; they were sometimes normal, suggesting that sensory symptoms related to metronidazole may be caused by a mixture of small-fiber and large-fiber sensory dysfunction.
Aseptic meningitis is a rare, possibly allergic adverse effect of metronidazole; the one published case was well documented, with a positive rechallenge. There has been a report of visual loss and headache after metronidazole.
A 68-year-old man with a tooth abscess had a tooth extraction and received amoxicillin. A few weeks later, he developed toothache again and was given amoxicillin and metronidazole 400 mg tds; he took no other drugs. Six hours after the first dose, he developed a headache. He continued with metronidazole for a total of three doses, and 6 hours after the last dose, the headache resolved. Two days later, he noticed flashing lights in both eyes. He then developed a central visual field defect and progressive visual loss. His blood pressure was 220/120 mmHg, but it settled spontaneously.
Visual acuity was 6/12 in both eyes, and fundoscopy showed marked disc swelling with hemorrhages without other features of hypertensive retinopathy. The full blood count, plasma viscosity, routine biochemistry, vasculitis screen, anticardiolipin antibodies, angiotensin-converting enzyme assay, chest X-ray, CT of the brain and orbits, MRI, and MRA were all normal. The CSF examination showed an opening pressure of 24 cm of water and 13 white cells/|j,l.
Over the next few months, his visual symptoms slowly improved, but he developed secondary optic atrophy. In this case, there was no other obvious cause for visual loss, and it could have been caused by metronidazole. The exact mechanism was unclear, but it may have been related to raised intracranial pressure. Convulsions occurred in an 87-year-old man who had taken metronidazole. Encephalopathy associated with metronidazole has been reported in a patient with chronic renal insufficiency.
A 58-year-old woman with end-stage renal insufficiency secondary to diabetic nephropathy developed abdominal wall cellulitis 4 days after insertion of a peritoneal dialysis catheter. She was given vancomycin, cefepime, and metronidazole in reduced doses (doses not stated) and 2 days later developed dysarthria, an intention tremor, dysmetria, and dysdiadochokinesia. “Routine” biochemical tests were unchanged and a CT scan of the brain was unremarkable, but an MRI scan showed cerebral and cerebellar atrophy with multifocal ischemic glial lesions. Metronidazole was withdrawn and 2 days later her symptoms and signs had completely resolved. It is difficult to ascribe encephalopathy unequivocally to metronidazole in this case.
Sensory Systems
Eyes
Acute myopia occurred after 11 days of treatment with metronidazole for Trichomonas infection; it resolved within 4 days after withdrawal but recurred on rechallenge. The combined figures of two major American reporting systems for adverse reactions listed seven cases of retrobulbar or optic neuritis associated with oral metronidazole; two had concurrent peripheral neuropathy. However, there is insufficient information to evaluate these reports.
Ears
Two reports of moderate to severe sensorineural deafness after metronidazole therapy, which resolved slowly after therapy ended, suggest that this is an additional adverse effect. Both cases of deafness were preceded by tinnitus, which may be a warning to withdraw the drug.
Hematologic
Metronidazole can produce leukopenia and neutropenia, usually only associated with prolonged therapy and reversible on withdrawal. One case each of agranulocytosis and aplastic anemia has been reported, and a single case of hemolytic-uremic syndrome in six children.
Gastrointestinal
Anorexia, nausea, vomiting, abdominal pain, and diarrhea have all been reported in patients taking metronidazole. The use of a large single dose most commonly leads to these complaints. A metallic taste also seems to be quite common, as is the occurrence of a black tongue. Although metronidazole is often used to treat pseudo-membranous colitis, it can also occasionally cause it.
Liver
Raised serum liver enzyme activities were reported in one case about 15 years ago, but there has been no more recent confirmation.
Pancreas
Pancreatitis has been reported in various individual case histories, but there is reason to think that some of these cases at least were due to other factors, such as alcohol use. Pancreatitis has been described in a 63-year-old woman with Crohn’s disease, coinciding with the administration of metronidazole and disappearing 1-2 days after withdrawal, but even here, there was little support for a causal link. Pancreatitis was attributed to metronidazole in a 61-year-old woman given intravenous metronidazole 500 mg 6-hourly. The relation between pancreatitis and metronidazole in this case was less convincing than in previously reported cases, as there was no rechallenge.
Urinary tract
Darkening of the urine can occur in patients taking metronidazole. This harmless discoloration is mostly seen during prolonged treatment.
Skin
Pruritus and rashes have been reported in patients taking metronidazole, including a fixed drug eruption and a pityriasis rosea-like eruption. Urticaria after a single dose has been reported but could have been coincidental In a case of fixed drug eruption, a provocation test showed cross-reactivity with tinidazole but not with secnidazole.
Reproductive system
A genital mucosal erosion occurred in a 38-year-old woman who had taken metronidazole 400 mg tds for 10 days for bacterial vaginitis.
Interactions
Check with your doctor or pharmacist before using your medicine if:
- you have or have ever had a liver problem;
- you are having kidney dialysis.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines. This includes medicines obtained without a prescription, including herbal medicines. This is because Metronidazole can affect the way some other medicines work. Also, some other medicines can affect the way Metronidazole works. In particular, tell your doctor if you are taking:
- anti-coagulants (blood thinning agents), such as warfarin. The dosage of warfarin may need to be reduced if you are taking this drug;
- lithium. If taken at the same time as Metronidazole [Flagyl], the kidneys may be affected;
- medicines used to treat epilepsy, such as phenytoin, primidone, and phenobarbitone;
- 5 fluorouracil for cancer;
- Busulfan for leukemia (cancer of the blood cells);
- Ciclosporin – to prevent the rejection of organs after transplant.
If you are not sure, talk to your doctor or pharmacist before taking Metronidazole.
| Drug/Interaction | Description |
|---|---|
| Alcohol | Metronidazole can cause a disulfiram-like reaction when taken with alcohol, leading to severe side effects. An unusual reaction was reported due to interaction with alcohol in X-Prep. |
| Antibiotics | In vitro studies indicate that metronidazole combined with antibiotics shows an additive effect against anaerobic bacteria. |
| Carbamazepine | Metronidazole may increase the toxicity of carbamazepine by inhibiting its metabolism. |
| Cephalosporins | High doses of metronidazole combined with cefamandole and clindamycin have been associated with encephalopathy. |
| Ciclosporin | Metronidazole can significantly increase blood concentrations of ciclosporin and tacrolimus, potentially leading to toxicity and increased serum creatinine levels. |
| Fluorouracil | Pretreatment with metronidazole may enhance the toxicity of fluorouracil; clinical significance is still under investigation. |
| Gentamicin | In guinea pigs, metronidazole augmented gentamicin-induced ototoxicity, as measured by compound action potentials. |
| Lithium | Metronidazole can increase the toxicity of lithium, necessitating careful monitoring. |
| Phenytoin | In patients on phenytoin, metronidazole’s hydroxylated metabolite concentration increased disproportionately, indicating phenytoin may induce metronidazole-metabolizing enzymes. |
| Quinidine | Co-administration of metronidazole can raise serum quinidine concentrations, likely due to inhibition of cytochrome P450 enzymes. |
| Tacrolimus | Similar to ciclosporin, metronidazole significantly increases blood concentrations of tacrolimus, posing a risk for toxicity in patients using these immunosuppressive drugs. |
| Vecuronium bromide | Metronidazole may potentiate the effects of non-depolarizing muscle relaxants; serum concentrations increased during concurrent administration with ciprofloxacin. |
| Warfarin and other coumarins | Metronidazole potentiates the effects of warfarin through stereoselective inhibition of 5-warfarin metabolism, increasing bleeding risk; similar interactions occur with acenocoumarol. |
Othe Brand Names and Dosages in Britain, the United States, and Canada
In pharmacies in the United States, Great Britain, and Canada, the pharmacists offer you Metronidazole under such brand names and in such strengths and dosage forms:
| UK | US | Canada |
| Flagyl 200mg Tablets
Flagyl 400mg Tablets Flagyl 500mg/100ml Solution Flagyl S Suspension Metrogel Metrolyl 1g Suppositories Metronidazole 200mg Tablets Metronidazole 400mg Tablets Metronidazole Tablets 500mg Norzol 200mg/5ml Oral Suspension Rozex Cream Rozex Gel Zidoval 7.5 mg/g Vaginal Gel Zyomet Gel |
Flagyl 250mg Tablets
Flagyl 375mg Capsules Flagyl 500mg Tablets Flagyl ER 750mg Tablets Flagyl I.V. 500mg Injectable Metrocream 0.75% Cream Metrogel 0.75% & 1% Gel Metronidazole 375mg Capsules Metronidazole 0.75% Cream Metronidazole 0.75% & 1% Gel Metronidazole 250mg Tablets Metronidazole 500mg Tablets Metronidazole 750mg Tablets Metronidazole 500mg/100ml Injectable |
Flagyl 500mg Tablets
Flagyl Cream 10% Flagystatin Vaginal OvulE 500mg Metrocream 0.75% Cream Metrogel 0.75% & 1% Gel Metronidazole 250mg Tablets Metronidazole 500mg Tablets Metronidazole 5mg/ml Injectable PMS-Metronidazole 250mg Tablets PMS-Metronidazole 500mg Tablets |
Storage
Keep your medicine in a safe place, out of the reach and sight of children. Store it in its original packaging to protect it from light. Do not use this medicine after the expiry date shown on the pack.
Ask your pharmacist how to dispose of medicines that are no longer required. Do not flush medicines down a toilet or sink or throw them out with your normal household rubbish. This will help protect the environment.
Further Information
Each tablet contains 200mg or 400mg of Metronidazole as the active substance. The other ingredients are povidone, magnesium stearate, colloidal anhydrous silica, and maize starch.
The tablets are off-white colored, round, biconvex, and uncoated, engraved with either ‘MZ 200’ (200mg) or ‘MZ 400’ (400mg) and a broken line on one side whilst plain on the other.
Metronidazole Tablets 200mg are available in containers of 7, 14, 15, 21, 28, 42, 56, 70, 84, 100, 250, 500, and 1000 tablets, as well as in bottles containing 50 tablets.
Metronidazole Tablets 400mg are available in containers of 28, 30, 50, 60, 84, 90,100,112,120, 140, 168,180, 500 and 1000 tablets.
They are also available in blister packs of 7, 14, 15, 21, 28, 42, 56, 70, and 84 tablets (not all pack sizes may be marketed).
| Dosage forms of Metronidazole: | |||
|---|---|---|---|
| Metronidazole 0.75% cream | Metronidazole powder | Danazol 50 mg capsule | Noritate 1% cream |
| Danazol 100 mg capsule | Flagyl 250 mg tablet | Metronidazole benz powder | Metrolotion topical 0.75% |
| Flagyl 375 mg capsule | Danazol 200 mg capsule | Metrocream 0.75% cream | Flagyl 500 mg tablet |
| MetroNIDAZOLE 750 mg 24 Hour tablet | Flagyl er 750 mg tablet | Flagyl ER 750 mg 24 Hour tablet | MetroNIDAZOLE 0.75% Gel 70 gm Tube |
| MetroNIDAZOLE 0.75% Gel 45 gm Tube | MetroNIDAZOLE 0.75% Cream 45 gm Tube | MetroNIDAZOLE 0.75% Lotion 59ml Bottle | Noritate 1% Cream 60 gm Tube |
| Metrogel 1% kit | Metrogel 1% Gel 60 gm Tube | Metrogel 1% Kit Box | MetroCream 0.75% Cream 45 gm Tube |
| MetroLotion 0.75% Lotion 59ml Bottle | Flagyl 5 mg/ml | Metro iv 500 mg/100 ml | Metronidazole 5 mg/ml |
| Metronidazole 500 mg/100 ml | Apo-Metronidazole 250 mg Tablet | Flagyl 10 % Cream | Metronidazole 250 mg tablet |
| Vandazole vaginal 0.75% gel | Metrogel-vaginal 0.75% gel | Metrocream 0.75 % Cream | Metrolotion 0.75 % Lotion |
| Rosasol 1 % Cream | Noritate 1 % Cream | Metrogel 1 % Gel | Metrogel 0.75 % Gel |
| Metronidazole 500 mg tablet | Metronidazole vaginal 0.75% gl | ||
Synonyms of Metronidazole
Methronidazole, Metronidazol, Metronidazole Benzoate, Metronidazole Hcl, Metronidazole In Plastic Container, Metronidazolo
Therapeutic Classes of Metronidazole
Anti-Infective Agents, Anti-Infectives, Antiprotozoal Agents, Antiprotozoals, Radiation-Sensitizing Agents



 (17 votes, average: 3.88 out of 5)
(17 votes, average: 3.88 out of 5)