Acute Pneumonias
Potential Severity
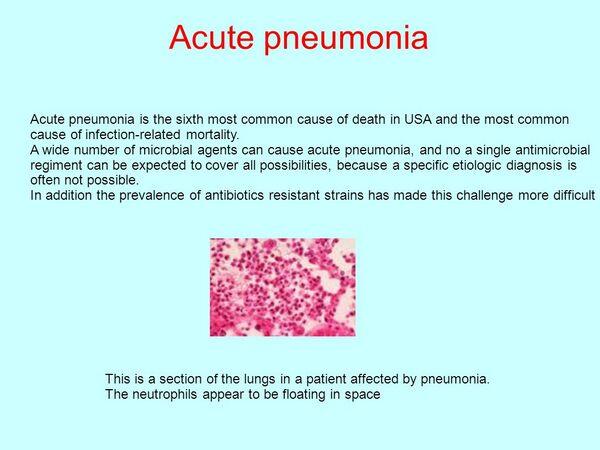
Acute pneumonia is a potentially life-threatening illness requiring rapid diagnosis and treatment. A delay in antibiotic treatment increases the risk of a fatal outcome.

General Considerations In Acute Pneumonia
Prevalence
Annually, 2 to 3 million cases of pneumonia are reported in the United States. Estimates suggest that pneumonia is responsible for more than 10 million physician visits, 500,000 hospitalizations, and 45,000 deaths annually. Overall, 258 people per 100,000 population require hospitalization for pneumonia, and that number rises to 962 per 100,000 among those over the of age 65 years. It is estimated that, annually, 1 in 50 people over 65 years of age and 1 in 20 over 85 years will develop a pneumonia. Pneumonia occurs most commonly during the winter months.
Causes
Improved diagnostic techniques have shown that the number of pathogens that cause acute pneumonia is ever expanding.
The leading cause of acute community-acquired pneumonia remains Streptococcus pneumoniae, followed by Haemophilus influenzae. Mycoplasma and Chlamydia pneumoniae also account for a significant percentage of acute pneumonias.
Table Common Causes of Acute Pneumonia
| Organism | Cases (%)a |
| Streptococcus pneumoniae | 16-60 |
| Haemophilus influenzae | 3-38 |
| Other gram-negative bacilli | 7-18 |
| Legionella spp. | 2-30 |
| Chlamydia pneumoniae | 6-12 |
| Mycoplasma | 1-20 |
| Staphylococcus aureus | 2-5 |
| Influenza A and В | — |
| Parainfluenza | — |
| Respiratory syncytial virus | — |
| Anaerobes (usually mixed) | — |
Staphylococcus aureus is an unusual community-acquired pathogen, but it can cause ventilator associated pneumonia. Gram-negative bacteria other than H. influenzae зхс also an uncommon cause of community-acquired pneumonia except in patients with underlying lung disease or alcoholism. Gram-negative pneumonia most commonly develops in hospitals or nursing homes. Legionella species vary in importance, depending on the season and geographic area. Anaerobes such as anaerobic streptococci and bacteroids can cause acute pneumonia following aspiration of mouth contents. Common viral pathogens include influenza, parainfluenza, and respiratory syncytial virus.
Pathogenesis and Pathology
Under normal conditions, the tracheobronchial tree is sterile. The respiratory tract has a series of protective mechanisms that prevent pathogens from gaining entry:
- The nasal passages contain turbinates and hairs that trap foreign particles.
- The epiglottis covers the trachea and prevents secretions or food from entering the trachea.
- The tracheobronchial tree contains cells that secrete mucin. Mucin contains a number of antibacterial compounds including immunoglobulin A antibodies, defensins, lysozymes, and lactoferrin. Mucin also is sticky, and it traps bacteria or other foreign particles that manage to pass the epiglottis.
- Cilia lining the inner walls of the trachea and bronchi beat rapidly, acting as a conveyer belt to move mucin out of the tracheobronchial tree to the larynx.
- When significant volumes of fluid or large particles gain access to the trachea, the cough reflex is activated, and the unwanted contents are quickly forced out of the tracheobronchial tree.
- If pathogens are able to bypass all of the above protective mechanisms and gain entry into the alveoli, they encounter a space that, under normal circumstances, is dry and relatively inhospitable. The presence of an invading pathogen induces the entry of neutrophils and alveolar macrophages that ingest and kill infecting organisms. Immunoglobulins and complement are found in this space. Surfactants also have a protective function.
- The lymphatic channels adjacent to the alveoli serve to drain this space and transport fluid, macrophages, and lymphocytes to the mediastinal lymph nodes.
Bacterial pathogens usually gain entry into the lung by aspiration of mouth flora or by inhalation of small aerosolized droplets (<3 um in diameter) that can be transported by airflow to the alveoli. Once the pathogen takes hold, a series of inflammatory responses is triggered. These responses have been most carefully studied in pneumonia attributable to S. pneumoniae.
First, an outpouring of edema fluid into the alveoli occurs, serving as an excellent culture media for further bacterial growth. As fluid accumulates, it spills over to adjacent alveoli through the pores of Kohn and the terminal bronchioles, resulting in a centrifugal spread of infection. Coughing and the physical motion of respiration further enhance spread.
About the Protective Mechanisms of the Lung
- Normally the tracheobronchial tree is sterile.
- The nasal turbinates trap foreign particles, and the epiglottis covers the trachea.
- Mucin has antibacterial activity, and cilia transport mucin out of the lung.
- Coughing expels foreign material that enters the tracheobronchial tree.
- Alveoli can deliver polymorphonuclear leukocytes, macrophages, immunoglobulins, and complement to destroy invading pathogens.
- Lymphatics drain macrophages and polymorphonuclear leukocytes to the mediastinal lymph nodes.
About the Pathogenesis of Pneumonia
- Pathogens are aspirated or inhaled as small aerosolized droplets.
- Bacterial invasion of the alveoli induces
- edema fluid that spreads to other alveoli through the pores of Kohn,and
- infiltration by polymorphonuclear leukocytes and red blood cells, followed by macrophages.
- Infection spreads centrifugally:
- Newer regions in the periphery appear red (“red hepatization”).
- Older regions are central and appear gray (“gray hepatization”).
- Streptococcal pneumonia does not cause permanent tissue destruction.
- Staphylococcus aureus, gram-negative rods, and anaerobes cause permanent damage.
Next, polymorphonuclear leukocytes and some red blood cells begin to accumulate in the alveolar space. Eventually, they fill the region and form a zone of consolidation.
Macrophages then enter the lesions and assist the polymorphonuclear leukocytes in clearing the infection. Histopathology reveals zones of varying age. The most distal regions represent the most recent areas of infection. There, edema fluid, polymorphonuclear leukocytes, and red blood cells predominant. On lower power microscopy, this region has an appearance similar to the architecture of the liver — an effect termed “red hepatization.” Older central regions have more densely packed polymorphonuclear leukocytes and macrophages. This region has a grayer color and forms the zone of “gray hepatization.”
Pulmonary pathogens demonstrate marked differences in their invasiveness and ability to destroy lung parenchyma. S. pneumoniae causes minimal tissue necrosis and is associated with little or no scar formation. Full recovery of pulmonary function is the rule. S. aureus releases a number of proteases that permanently destroy tissue. Gram-negative rods and anaerobic bacteria also cause permanent tissue destruction.
Predisposing Factors
Most bacterial pneumonias are preceded by a viral upper respiratory infection. Influenza virus is well known to predispose to S. pneumoniae and S. aureus pneumonia. Viral infections of the upper respiratory tract can damage the bronchial epithelium and cilia.
Virus-mediated cell damage also results in the production of serous fluid that can pool in the pulmonary alveoli, serving as an excellent culture media for bacteria. The low viscosity of this fluid, combined with depressed ciliary motility enables the viral exudate to carry nasopharyngeal bacteria past the epiglottis into the lungs. Smoking also damages the bronchial epithelial cells and impairs mucociliary function. As a consequence, smokers have an increased risk of developing pneumonia. Congenital defects in ciliary function (such as Kartagener’s syndrome) and diseases resulting in highly viscous mucous (such as cystic fibrosis) predispose patients to recurrent pneumonia.
An active cough and normal epiglottal function usually prevent nasopharyngeal contents from gaining access to the tracheobronchial tree. However, drugs such as alcohol, sedatives, and anesthetics can depress the level of consciousness and impair these functions, predisposing the patient to pneumonia. Elderly individuals, particularly after a cerebrovascular accident, often develop impairments in swallowing that predispose them to aspiration. In addition, elderly people demonstrate reduced humoral and cell-mediated immunity, rendering them more susceptible to viral and bacterial pneumonia.
About Factors that Predispose to Pneumonia
- Viral infections damage cilia and produce serous exudate that can transport nasopharyngeal bacteria into the alveoli.
- Smoking damages bronchial epithelial cells and impairs ciliary function.
- Alcohol and other drugs depress coughing and epiglottal function.
- Elderly patients have reduced humoral and cell-mediated immunity, and may have impaired swallowing because of stroke.
- Patients on immunosuppressive agents and patients with AIDS have depressed humoral and cell-mediated immunity.
- Patients with chronic diseases are at increased risk for pneumonia.
- Cold weather dries the mucous membranes and increases person-to-person spread of infection.
Patients with impairments in immunoglobulin production, T- and B-cell function, and neutrophil and macrophage function are also at greater risk for developing pneumonia. Organ-transplant patients on immunosuppressive agents and AIDS patients have a greater likelihood of developing pneumonia. Chronic diseases, including multiple myeloma, diabetes, chronic renal failure, and sickle cell disease have been associated with an increased risk of pneumonia.
Cold weather is thought to contribute to the development of pneumonia. Cold, dry weather can alter the viscosity of mucous and impair bacterial clearance. Cold weather also encourages people to remain indoors, a situation that enhances person-to-person spread of respiratory infections.
Symptoms and Signs
Case 1
A 55-year-old woman was first seen in the emergency room in December complaining of a nonproductive cough, nasal stuffiness, and fever. She also noted diffuse severe muscle aches and joint pains and a generalized headache. In her epidemiologic history, she noted that she had recently seen her grandchildren, who all had high fevers and were complaining of muscle aches.
Physical exam showed these positive findings: temperature, 39°C; throat, erythematous; nasal discharge, clear; muscles, diffusely tender. A chest x-ray (Chest X-Ray) was within normal limits.
Three days into the clinical course of her illness, the patient noted some improvement in her cough, muscle aches, and joint pains; however, on the 4th day, she developed a high fever (40°C) preceded by a teeth-chattering chill. That day, her cough became productive of rusty-colored opaque sputum, and she began feeling short of breath.
A repeat physical exam showed a temperature of 40.6°C and a respiration rate of 36 per minute. In general, this was a very ill-appearing, anxious woman, gasping for air. Lungs were mildly dull to percussion, with E-to-A changes, and rales and rhonchi localized to the left lower lobe.
A peripheral white blood cell count measured 16,000/mm3, with 68% polymorphonuclear leukocytes, 20% immature forms (bands and metamyelocytes), 8% lymphocytes, and 4% monocytes. Sputum Gram stain showed many gram-positive lancet-shaped diplococci, many polymorphonuclear leukocytes (>10/high-power field), and no squamous epithelial cells. A Chest X-Ray revealed a dense left lower lobe and lobar infiltrate.
In case 1, the patient’s initial symptoms suggested a viral illness involving the upper respiratory tract (rhinitis and nonproductive cough); central nervous system or air sinuses, or both (headache); and musculoskeletal system (myalgias and arthralgias). Such symptoms are generally attributed to an influenza-like illness. A number of viruses can explain these symptoms, including influenza, parainfluenza, adenovirus, respiratory syncytial virus (more common in children, but also found in elderly individuals and transplant patients), rhinoviruses (usually less severe), and enteroviruses.
Subsequently, within a 24-hour period, this patient experienced the abrupt onset of a new constellation of symptoms. The onset of the new illness can be classified as acute. An illness is termed “acute” when symptoms and signs develop over 24 to 48 hours. Symptoms that develop over 3 days to 1 week are generally classified as subacute, and symptoms that progress more slowly (over 3 weeks to several months) are classified as chronic.
In generating a potential list of causative agents, the infectious disease specialist frequently uses the pace of the illness to narrow the possibilities. Pneumonias are generally classified into two groups: acute and chronic. Most bacterial and viral pneumonias develop quickly; fungal and mycobacterial pulmonary infections tend to develop at a slower pace. Acute pneumonia can be further classified as “typical” or “atypical.” Typical pneumonia is characterized by the more rapid onset of symptoms, more severe symptomatology, a productive cough, and dense consolidation on Chest X-Ray, as observed in case 4.1. Atypical pneumonia tends to be slower in onset (often subacute), symptoms tend to be less severe, cough is productive of minimal sputum, and Chest X-Ray usually reveals a patchy or interstitial pattern. Finally, pulmonary infections are separated into community-acquired or nosocomial. “Community-acquired” is defined as an infection developing in a patient who has not recently (>14 days) been hospitalized or resided in a chronic care facility.
Although considerable overlap in symptoms, signs, and Chest X-Ray findings are observed in cases of acute community-acquired pneumonia, certain key clinical characteristics are helpful in guiding the determination of the most likely causes. Generation of a logical differential list of potential pathogens guides the choice of diagnostic tests and narrows the possible treatment regimens.
Important symptoms that need to be reviewed include these:
- Cough. Frequency of the cough, production of sputum, and color of the sputum should be documented. A nonproductive cough or a cough productive of scanty sputum suggests an atypical pneumonia; a cough productive of rusty-colored sputum raises the possibility of S. pneumoniae. Thick, “red current jelly” sputum has been reported in cases of Khbsiellapneumoniae, green-colored sputum is more frequently encountered in patients with H. influenzae and Pseudomonas aeruginosa pneumonia (typically a nosocomial pathogen, or found in patients cystic fibrosis). Frank hemoptysis is observed in cavitary tuberculosis, lung abscess, and lung carcinoma. It should be emphasized that considerable overlap occurs in the sputum characteristics of the various forms of pneumonia, and these observations cannot be considered specific.
- Chest discomfort. Pleuritic chest pain (pain associated with deep inspiration) is classically described in patients with S. pneumoniae. Pain is usually sharp and stabbing. Because the pulmonary parenchyma has no pain-sensing nerves, the presence of chest pain indicates inflammation of the parietal pleura. When the diaphragm becomes inflamed, the pain can mimic cholecystitis or appendicitis, and on occasion this sort of pain has precipitated exploratory laparotomy. Anaerobes, S. pyogenes, and S. aureus are other pathogens that can also spread to the pleura and cause chest pain. Pleuritic pain is also characteristic of pleurodynia, a pain syndrome caused by the enteroviruses coxsackievirus and echovirus.
- Rigor. Mild chills are encountered in most febrile illnesses. However, a teeth-chattering, bed-shaking chill indicative of a true rigor is usually associated with bacteremia. This symptom is very prominent, and patients often can report the exact time of their first rigor. A single rigor is the rule in pneumococcal infection; multiple rigors are more typical ofS. aureus, anaerobes, Kbbsiella species, and S. pyogenes. H. influenzae seldom causes rigors.
- Shortness of breath. A report of increased shortness of breath suggests poor alveolar oxygen exchange, indicative of severe infection. Some patients experience shortness of breath as a result of pleuritic chest pain that limits the ability to breath deeply. To avoid pain, patients may breath quickly and shallowly, and this breathing pattern may be interpreted as shortness of breath.
- Epidemiology. A careful epidemiologic history is often helpful. A number of environmental factors predispose to pneumonia. Animal exposure must be carefully reviewed, including contact with wild game, birds, bats, and rodents. Exposure to outside air conditioning units or construction sites should be identified (legionnaires’ disease). Travel history may be helpful. For example, travel to the Southwest raises concerns about coccidiomycosis, and travel to the Ohio River valley raises the possibility of histoplasmosis. Because many respiratory illnesses spread from person to person, a history of exposure to family members or friends with illnesses should be ascertained. Occupational and sexual history should also be elicited.
About the Classification of Pneumonia Pneumonias are classified by
- pace of illness:
- Acute — symptoms develop over 24 to 48 hours.
- Chronic — symptoms progress over 3 weeks or longer.
- specific constellations of symptoms:
- Typical — rapid onset, more severe symptoms, productive cough,dense consolidation on chest X-ray (Chest X-Ray).
- Atypical — somewhat slower onset, less severe symptoms, nonproductive cough, patchy interstitial pattern on Chest X-Ray.
- environment in which the pneumonia was acquired:
- Community-acquired — patient not recently (>14 days) in a hospital or chronic care facility.
- Nosocomial — patient in a hospital at the time the infection developed.
Table Clinical Characteristics of Acute Community-Acquired Pneumonia Classified by Cause
| Causative agent | Classical symptoms | Typical radiographic findings |
| Streptococcus pneumoniae | Rusty-colored sputum, rigor, pleuritic chest pain | Lobar infiltrate, air bronchograms |
| Haemophilus influenzae | More gradual onset; seen in smokers with COPD | Lobar or patchy infiltrates |
| Staphylococcus aureus | Follows influenza pneumonia, rapidly progressive acute disease | Bronchopneumonia, lung abscess, pneumothorax,and empyema |
| Aspiration pneumonia | Follows from loss of consciousness, poor gag reflex, abnormal swallowing; foul-smelling sputum | Dense consolidation (more in the right lower lobe than in the left lower lobe, or in posterior segment of upper lobes); later, lung abscess and empyema |
| Legionella pneumophila | Nonproductive cough, gastrointestinal symptoms, confusion | Lobar pneumonia,cavities in immunocompromised patients |
| Atypical pneumonia | Mild-to-moderate symptoms, nonproductive cough, pulmonary exam often normal | Patchy lower lobe bronchopneumonia |
Key Points
About the History in Pneumonia
- Cough. Frequency, production of sputum, color and thickness of sputum.
- Chest pain. Pain on deep inspiration, usually sharp, suggests pleural involvement. Seen in Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus pyogenes, anaerobes, and coxsackievirus and echovirus.
- Rigor. Bed-shaking chills, 1 rigor in S. pneumoniae, more than 1 in S. aureus, Klebsiella spp., S. pyogenes, and anaerobes.
- Shortness of breath. A worrisome symptom, may be the result of pleuritic chest pain rather than poor gas exchange.
- Epidemiology. Travel history, animal exposure, exposure to people with respiratory illnesses, occupational and sexual history.
About the Physical Exam in Pneumonia
- A respiratory rate >30/min, a blood pressure <90 mm Hg,a pulse >125/min,and a temperature <35°C or >40°C are bad prognostic findings.
- Depressed mental status and stiff neck suggest bacterial meningitis.
- Pulmonary auscultation often underestimates the extent of pneumonia:
- Bronchial breath sounds and egophony suggest consolidation.
- Dullness to percussion indicates consolidation or a pleural effusion.
- Pleural effusion is accompanied by decreased breath sounds and, in some cases, a friction rub.
A thorough physical examination should be performed during the initial evaluation for possible pneumonia. Vital signs are helpful in determining the severity of illness. A respiratory rate of more than 30 breaths per minute, a systolic blood pressure under 90 mm Hg, a pulse above 125 beats per minute, and a temperature below 35°C (95°F) or above 40°C (104°F) are all bad prognostic signs. Depressed mental status is also associated with a poor prognosis.
Ear, nose, and throat examination may reveal vesicular or crusted lesions consistent with Herpes labialis, an infection that may reactivate as a consequence of the stress of the primary illness. Neck stiffness in association with depressed mental status may indicate the development of bacterial meningitis, a potential complication of pneumococcal pneumonia.
Pulmonary auscultation often fails to detect the extent of infection, and when pneumonia is being considered, the physical exam should be followed by a Chest X-Ray. Asymmetry of chest movements may be observed, movement being diminished on the side with the pneumonia. When infection has progressed to consolidation, filling of the lung parenchyma with exudate alters sound conduction. Air flow from the bronchi is conducted through this fluid to the chest wall, resulting in bronchial or tubular breath sounds. When the patient is asked to say “E,” an ‘A” is heard on auscultation (egophony). Percussion of the chest wall also demonstrates dullness in the areas of consolidation. Dullness to percussion in association with decreased breath sounds suggests the presence of a pleural effusion. A “leathery” friction rub may be heard over the site of consolidation, indicating pleural inflammation.
Laboratory Findings
Physical examination is unreliable for making the diagnosis of pneumonia. If pneumonia is a potential diagnosis, Chest X-Ray must to be performed to confirm or exclude the disease. The radiologic pattern can serve as a rough guideline to possible causative agents; however, the use of immunosuppressive agents (resulting in neutropenia, decreased cell-mediated immunity, and depressed macrophage function) can greatly alter the typical radiologic appearance of specific pathogens. Patients with AIDS also present with atypical Chest X-Ray. Five classical patterns have been described:
- Lobar pneumonia. “Lobular pneumonia” refers to a homogeneous radiologic density that involves a distinct anatomic segment of the lung. Infection originates in the alveoli. As it spreads, this form of infection respects the anatomic boundaries of the lung and does not cross the fissures. Lobar pneumonia is most commonly seen with S. pneumoniae, H. influenzae, and Legionella.
- Bronchopneumonia. The bronchopneumonia form of pulmonary infection originates in the small airways and spreads to adjacent areas. Infiltrates tend to be patchy, to involve multiple areas of the lung, and to extend along bronchi. Infiltrates are not confined by the pulmonary fissures. Bronchopneumonia is commonly observed with S. aureus, gram-negative bacilli, Mycoplasma, Chlamydia, and respiratory viruses.
- Interstitial pneumonia. Infections causing inflammation of the lung interstitium result in a fine diffuse granular infiltrate. Influenza and cytomegalovirus commonly present with this Chest X-Ray pattern. In AIDS patients, Pneumocystis jirovecii infection results in interstitial inflammation combined with increased alveolar fluid that can mimic cardiogenic pulmonary edema. Miliary tuberculosis commonly presents with micronodular interstitial infiltrates.
- Lung abscess. Anaerobic pulmonary infections often cause extensive tissue necrosis, resulting in loss of lung tissue and formation of cavities filled with inflammatory exudate. S. aureus also causes tissue necrosis and can form cavitary lesions.
- Nodular lesions. Histoplasmosis, coccidiomycosis, and cryptococcosis can present as nodular lung lesions (multiple or single) on Chest X-Ray. Hematogenous pneumonia resulting from right-sided endocarditis commonly presents with “cannonball” lesions that can mimic metastatic carcinoma.
About Chest X-Ray in Pneumonia
- If pneumonia is being considered, a chest X-ray (Chest X-Ray) should always be performed.
- Radiographic patterns may be atypical in patients receiving immunosuppressants and in patients with AIDS.
- Five typical Chest X-Ray patterns have been described:
- Lobar pattern. Streptococcus pneumoniae, Haemophilus influenzae,and Legionella.
- Bronchopneumonia pattern. Staphylococ-cus aureus, gram-negative organisms, Mycoplasma, Chlamydophila, and viral.
- Interstitial pattern. Influenza and cytome-galovirus,Pne(jmocysf/s, miliary tuberculosis.
- Lung abscess. Anaerobes, S. aureus.
- Nodular lesions. Fungal (histoplasmosis, coccidiomycosis, cryptococcosis) and right-sided endocarditis.
- Patterns on chest radiographs are only rough guides. Considerable overlap between the various pathogens has been observed.
The role of high-resolution chest computed tomography scan is evolving, and this test has proved helpful for more clearly demonstrating interstitial infiltration, pulmonary cavities, nodules, and pleural fluid collections.
Patients with an infiltrate, who are under age 65, have a normal mental status, and normal or only mildly deranged vital signs can be treated as outpatients. Sputum Gram stain and culture are optional in these patients, as are any additional tests.
In more severely ill patients who are being considered for hospitalization, additional tests to assess the severity of the illness need to be ordered.
A complete and differential blood cell count should be obtained. Patients with bacterial pneumonia usually have an elevated peripheral white blood cell count and a left shift. When pneumococcal pneumonia is accompanied by a low peripheral white blood cell count (<6000), a fatal outcome is more likely. The finding of anemia (hematocrit <30%), usually indicative of chronic underlying disease, is also associated with a worse prognosis.
Blood oxygenation also needs to be assessed. The 02 saturation should be determined, and if it is at all depressed, an arterial blood gas should be obtained. Systemic acidosis (pH <7.35) and an arterial partial pressure below 60 mm Hg are bad prognostic signs. A significant depression in oxygenation reflects loss of alveolar function and lack of oxygen transfer to alveolar capillaries. Deoxygenated blood passes from the right side of the heart to the left side, creating a physiologic right-to-left shunt.
Other metabolic parameters also need to be assessed. A blood urea nitrogen level above 30 mg/dL reflects hypoperfusion of the kidneys or dehydration (or both) and is a negative prognostic finding. A serum sodium reading below 130 mEq/L reflects increased antidiuretic hormone secretion in response to decreased intravascu-lar volume in addition to severe pulmonary disease. Such a reading is another negative prognostic finding, as is a serum glucose level exceeding 250 mg/dL. Two blood cultures should be drawn before antibiotics are started. Positive blood cultures definitively identify the cause of the disease. Blood cultures are positive in 1% to 16% of cases of community-acquired pneumonia.
Sputum requires careful analysis and frequently provides helpful clues to the probable diagnosis. Sputum samples often become contaminated with bacteria and cells from the nasopharynx, making interpretation of the cultures difficult. Ideally, acquisition of sputum should be supervised by a physician to ensure that the patient coughs deeply and brings up the sample from the tracheobronchial tree and does not simply expectorate saliva from the mouth. The adequacy of the sample should be determined by low-power microscopic analysis of the sputum Gram stain. The presence of more than 10 squamous epithelial cells per low-power field indicates significant contamination from the nasopharynx, and the sample should be discarded. The presence of more than 25 polymorphonuclear leukocytes per low-power field and the presence of bronchial epithelial cells provide strong evidence that the sample originates from the tracheo-bronchial tree.
About Blood Tests in Pneumonia
- With the exception of patients under the age of 50 years, without underlying disease, and with normal vital signs, multiple blood tests are used to assess the severity of disease.
- A peripheral white blood cell count below 6000/mm3 in Streptococcus pneumoniae is a bad prognostic finding.
- Anemia (hematocrit <30%),blood urea nitrogen above 30 mg/dL, serum sodium below 130 mEq/L, and glucose above 250 mg/dL are associated with a worse prognosis.
- Arterial blood o2 below 60 mm Hg and pH below 7.35 worsen prognosis.
- Two blood samples should be drawn before antibiotics are stated; blood cultures are positive in up to 16% of patients.
Despite originating from deep within the lungs, sputum samples usually become contaminated with some normal throat flora as they pass through the nasopharynx. Gram stain can be helpful in differentiating normal flora (mixed gram-positive and gram-negative rods and cocci) from the offending pathogen. When a single bacterial type predominates, that bacterium is likely to be the primary pathogen. For example, the presence of more than 10 lancet-shaped gram-positive diplococci per high-power field provides strong evidence that S. pneumoniae is the cause of the pneumonia (approximately 85% specificity and 65% sensitivity).
In reviewing bacterial morphology, the observer must assess the adequacy of decolorization. In ideally stained regions, the nucleus and cytoplasm should be gram-negative, and a mixture of gram-positive and gram-negative organisms should be seen. A gram-positive nucleus indicates underdecolorization, and the presence of gram-negative bacteria only (including cocci) suggests overdecolorization.
Sputum Gram stain is also helpful for assessing the inflammatory response. The presence of many polymorphonuclear leukocytes suggests a bacterial cause for the disease; a predominance of mononuclear cells is more consistent with Mycoplasma, Chlamydia, or a viral infection.
Sputum culture is less helpful than Gram stain, because normal flora contaminating the sample frequently overgrow, preventing identification of the true pathogen. To reduce overgrowth, samples should be quickly inoculated onto culture media. Rapid processing has been shown to increase the yield for S. pneumoniae. Sputum cultures are falsely negative approximately half the time. Because of the potential problems with sampling error, and the inability to accurately quantify bacteri by standard culture, sputum should never be cultured in the absence of an accompanying Gram stain.
Culture is most helpful in determining the antibiotic sensitivities of potential pathogens. The combination of sputum Gram stain and antibiotic sensitivity testing may allow the clinician to narrow the spectrum of antibiotic coverage, reducing the likelihood of selecting for highly resistant pathogens. In the intubated patient, sputum culture alone should never be the basis for initiating antibiotic therapy. Sputum culture will almost always be positive, a result that often simply represents colonization and not true infection.
Additional methods for sputum analysis are being developed. Polymerase chain reaction is being used to amplify specific strands of Deoxyribonucleic acid from pathogens.
About Sputum Gram Stain and Culture
- Ideally the sputum collection should be supervised by a physician.
- Adequacy of the sample is assessed by low-power microscopic analysis:
- More than 10 squamous epithelial cells indicates extensive contamination with mouth flora.
- More than 25 polymorphonuclear leukocytes or bronchial epithelial cells (or both) per low-power field indicate an adequate sample.
- Sputum Gram stain should be performed in all seriously ill patients with pneumonia.
- Decolorization should be assessed for adequacy.
- Predominance of a single organism suggests that the probable pathogen has been found.
- Predominance of polymorphonuclear leukocytes suggests bacterial pneumonia.
- Predominance of mononuclear cells suggests Mycoplasma, Chlamydophila, or a virus.
- Sputum culture
- Should never be ordered without an accompanying Gram stain.
- Should not be the sole basis for antibiotic treatment.
- Often represents colonization rather than infection when positive in the intubated patient.
- is insensitive, because mouth flora can overgrow the pathogen.
- Is helpful for determining the antibiotic sensitivity of pathogens identified by Gram stain.
This method will be particularly helpful in identifying organisms that are not normally part of the mouth flora and that are difficult to culture: L. pneumophila, Mycoplasma pneumoniae, Chlamydia pneumoniae, and P. jirovecii.
When Legionella pneumonia is a consideration (see specific discussion later in this chapter), urinary antigen for L. pneumophila serogroup 1 (the most common pathogenic serogroup) should be performed. This test is moderately sensitive and highly specific. A positive test is therefore diagnostic; a negative test does not exclude the diagnosis, however. A urinary antigen test for S. pneumoniae is also available and is recommended as potentially useful in adults (80% sensitivity for bacteremic patients, 97% specificity). This test is frequently positive in children colonized with S. pneumoniae, and is therefore not recommended for that patient population.
More invasive procedures are usually not required in community-acquired pneumonia, but may be considered in the severely ill patient when an adequate sputum sample cannot be obtained. Invasive procedures such as fiberoptic bronchoscopy with protected brushing or lavage are more commonly required in the immuno-compromised patient. The sheath surrounding the brush reduces, but does not eliminate, contamination by mouth flora.
Quantitative cultures are required to differentiate infection from contamination, with growth of more than 103 to 10 organisms per millihter indicating infection. Lavage of a lung segment with sterile fluid samples a larger volume of lung and is particularly useful for diagnosing P. jirovecii pneumonia in AIDS patients. Bronchoscopy has been shown to be useful in diagnosing not only P. jirovecii, but also mycobacterial infections and cytomegalovirus.
The use of bronchial lavage to assist in the diagnosis of ventilator-associated pneumonia is controversial. Contamination of samples by organisms colonizing the endotracheal tube can result in misinterpretation of the quantitative cultures. As compared to samples derived from endotracheal suction, samples obtained by bronchoscopy offer no benefit in regard to morbidity, mortality, or reduction in antibiotic use in ventilator-associated pneumonia.
Deciding On Hospital Admission In Acute Pneumonia
Specific Causes Of Acute Community-Acquired Pneumonia
Chronic Pneumonias Tuberculosis
Potential Severity
The miliary form of the tuberculosis can be fatal. Clinicians must maintain a high index of suspicion for tuberculosis in immigrants, indigent and elderly patients, and patients with AIDS.
Case 3
A 73-year-old black man, a retired bartender, came to the emergency room complaining of increasing shortness of breath and worsening cough over the preceding 3 weeks. About 5 months earlier, he had begun to notice night sweats that drenched his pajamas. That symptom was followed by development of a nonproductive cough. He began bringing up small guantities of yellow sputum 1 month before presentation at the emergency room. At the time that he noticed the sputum production, he began experiencing increased shortness of breath, even after mild exertion (walking 2 blocks to the grocery store). During the past few months, he felt very tired, and he has lost 10pounds despite a “good”diet.
Epidemiologic history indicated city residence and visits with a number of old drinking buddies. The patient denied exposure to anyone with tuberculosis, and he had no family history of tuberculosis.
Past medical history revealed an abnormal Chest X-Ray 20 years earlier and treatment at New York City’s Bellevue Hospital with isoniazid (INH) and para-aminosalicylic acid for 1 year.
Social history indicated that the patient had recently retired after 35 years of tending bar. He lives alone in a 1-bedroom apartment and supports himself on Social Security. He is a former smoker (half a pack daily for 28 years) and drinks half a pin t daily.
On physical exam, his temperature was 38°C and his respiratory rate was 18 per minute, presenting a picture of a thin male breathing comfortably. Aside from mild clubbing of his nail beds, the physical findings (including lung exam) were within normal limits.
The laboratory workup showed a hematocrit of 39% and a white blood cell count of 1OOO/mm3, with 55% polymorphonuclear leukocytes, 30%> lymphocytes, and 15%> monocytes.
Sputum Gram stain revealed many polymorphonuclear leukocytes, few gram-positive cocci, and rare gram-negative rods.
Bilateral upper lobe cavitary lesions were observed on Chest X-Ray. Acid-fast stain of the sputum revealed multiple acid-fast bacilli per high-power field.
Pathogenesis
Mycobacterium tuberculosis is an aerobic, nonmotile bacillus with a waxy lipid-rich outer wall containing high concentrations of mycolic acid. This waxy outer wall fails to take up Gram stain. Visualization of mycobacteria requires heating to melt the outer wall, which allows for penetration and binding of the red dye fuchsin. The lipids in the cell wall bind this dye with high affinity and resist acid-alcohol decolorization. This acid-fast bacillus is small in size and appears beaded. Genomic analysis reveals that, as compared with other bacteria, M. tuberculosis has a large number of genes encoding for enzymes that regulate
lipogenesis and lipolysis. The resulting high lipid content of this pathogen accounts for many of its unique clinical characteristics, including its ability to resist killing by macrophages and polymorphonuclear leukocytes and to survive for many years within the body. Rate of growth in M. tuberculosis is very slow, being about l/20th the growth rate of most conventional bacteria. The slow rate of growth may also be explained by the waxy cell wall, which limits access to nutrients.
Mycobacteria survive and grow in macrophages, and they therefore induce a profound chronic inflammatory response. On gaining entry to the lungs, these organisms are ingested by alveolar macrophages and transported to the hilar lymph nodes. Here macrophages and dendritic cells present tubercular antigens to T cells, inducing a cell-mediated immune response. Helper T cells (CD4 + ) then activate macrophages to kill the mycobacteria and control the infection. Accumulation of one of the cell wall waxes, cord factor, stimulates the formation of granulomas that contain clusters of epithelioid cells, giant cells, and lymphocytes. Over time, the centers of the granulomas become necrotic, forming cheesy debris called caseous necrosis. Caseating granulomas are the hallmark lesion of tuberculosis. This pathologic finding is only rarely found in other diseases. If intracellu-lar growth of M. tuberculosis continues, increasing numbers of macrophages are activated to produce multiple cytokines. Interleukin 1 stimulates the hypothal-amus to raise core body temperature, causing fever. Tumor necrosis factor interferes with lipid metabolism and causes severe weight loss. These cytokines are primarily responsible for the symptoms of fever, night sweats, and weight loss described in case 3.
Epidemiology
Humans are the only reservoir for M. tuberculosis. Person-to-person spread of infection is almost exclusively caused by inhalation of droplet nuclei that have been aerosolized by coughs or sneezes. The likelihood of inhaling infectious droplets is greatly increased in a closed, crowded environment. A single cough has been estimated to form 3000 infectious droplets, with a sneeze producing even higher numbers.
The infectiousness of an individual patient can be estimated by acid-fast bacilli smears. The higher the number of organisms per microscopic field, the greater the infectious potential. Patients with laryngeal tuberculosis are particularly infectious and can release large numbers of organisms while speaking. Patients with AIDS and tuberculosis often harbor a large organism burden. Patients with large pulmonary cavities tend to intermittently release large numbers of infectious particles.
Repeated exposure and close contact are generally required to contract this disease. Respiratory isolation and rapid treatment of infected individuals are the primary ways to prevent spread of infection.
Despite the availability of antituberculous agents, tuberculosis remains a leading cause of death worldwide. Crowded living conditions and the existence of immunologically naive populations continue to allow rapid person- to-person spread, particularly in underdeveloped countries. After a surge in cases in the United States during the mid-1980s because of the AIDS epidemic, the case rate has steadily declined. In 2002, it reached the lowest level ever recorded: 5.2 cases per 100,000. This steady decline among permanent U.S. residents contrasts with the steady increase in the percentage of tuberculosis cases among people immigrating to the United States. Immigrants now account for half of all reported cases in the United States. Individuals immigrating from underdeveloped countries have higher rates of infection. For example, the rate among Vietnamese immigrants is 120 per 100,000 person-years, and among Haitian immigrants it is 133 per 100,000. Immigrants from established market economies such as those of Western Europe have rates similar to those in the United States.
About the Pathogenesis of Tuberculosis
- Slow-growing aerobic rod, not seen on Gram stain.The lipid-rich outer wall binds the red dye fuchsin, which is not removed by acid, making the bacterium acid-fast.
- The lipid wall also allows the bacterium to resist drying and many disinfectants. It further allows the bacterium to survive within macrophages for years.
- Macrophages carry the mycobacterium to the lymph nodes, where a cell-mediated immune response is generated.
- Caseating granulomas are formed as a consequence of the cell-mediated immune response and the accumulation of lipid-rich bacteria.
- Increased levels of interleukin 1 cause fever, and increased levels of tumor necrosis factor cause weight loss.
About the Epidemiology of Tuberculosis
- Humans are the only reservoir of this disease.
- Person-to-person spread occurs via aerosolized infectious droplets from sneezes or coughs.
- Laryngeal tuberculosis is highly infectious.
- Patients with HIV release large numbers of organisms.
- Large cavitary lesions are also highly infectious.
- People with these characteristics are at increased risk:
- Immigrants from developing countries
- Alcoholics
- Urban poor
- Single men
- Intravenous drug abusers
- Migrant farm workers
- Prison inmates
- People infected with HIV
- Elderly people
- A genetic predisposition is found in people who are black, Hispanic, Asia Pacific Islanders, and Native Americans (5 to 10 times the incidence seen in Caucasians)
Tuberculosis occurs more frequently in single men, alcoholics, intravenous drug abusers, the urban poor (particularly homeless people), migrant farm workers, and prison inmates. Elderly people are more likely to develop secondary tuberculosis because cell-mediated immunity wanes with age.
A genetic predisposition to the development of active tuberculosis is known. People with European heritage tend to be more resistant, probably as a consequence of the devastating effects of the tuberculosis epidemic during the Industrial Revolution. At that time, tuberculosis was responsible for one fourth of the deaths in Europe, killing off a significant percentage of the population that had a reduced immune response to mycobacteria.
About Primary Tuberculosis
- Represents the first exposure to inhaled infectious particles.
- Followed by a flu-like illness.
- Spread is controlled over 4 to 8 weeks by the development of cell-mediated immunity.
- Ghon foci are calcified lung lesions at the site of the primary infection.
- Bacteremia develops and seeds the kidneys, epiphyses of the long bones,and vertebral bodies (areas with high oxygen content).The infection can later reactivate.
As compared with white people, people who are black or Hispanic, or who are Asia Pacific Islanders or Native Americans experience a 5 to 10 times higher incidence of tuberculosis. Patients with AIDS are particularly susceptible to tuberculosis, and this population has spread the infection to others. Areas and demographic groups in which AIDS is more prevalent therefore have a higher incidence of tuberculosis.
The patient in case 3 has a number of epidemiologic characteristics that increase his risk for tuberculosis. He is a single male, black, possibly alcoholic, and elderly.
Clinical Manifestations
There are two forms of human tuberculosis infection: primary tuberculosis and secondary tuberculosis.
Primary Tuberculosis
Primary disease occurs when a patient inhales infectious M. tuberculosis droplets for the first time. A flu-like illness usually follows; however, some people experience no symptoms. Within 4 to 8 weeks of exposure, the human host usually mounts a cell-mediated immune response. Activated macrophages control the spread and growth of the organism. Pulmonary lesions heal spontaneously and form areas of fibrosis or calcification called Ghon lesions or foci. A Ghon lesion in combination with hilar adenopathy is called a Ranke complex.
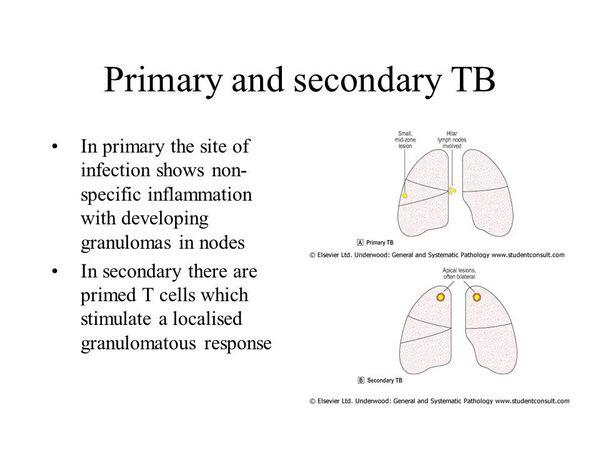
In addition to transporting organisms to the hilum and mediastinum, infected macrophages may gain access to the thoracic duct, enter the bloodstream, and spread throughout the body. M. tuberculosis grows best in regions with high oxygen tension, including the kidneys, long-bone epiphyses, and vertebral bodies. It most commonly infects the apices of the lung, the regions with the highest oxygen content and reduced lymphatic flow.
Although the infection is brought under control, the bacilli are not usually completely eradicated. Organisms can survive for decades, being held in check by the host immune response. But any condition that subsequently depresses cell-mediated immunity can free M. tuberculosis to grow and cause symptomatic secondary tuberculosis.
Miliary Tuberculosis
In some individuals, initial exposure to M. tuberculosis fails to induce cell-mediated immunity, or the immune response is not robust enough to control the infection. Under these conditions, the mycobacteria continue to multiply and disseminate, causing miliary tuberculosis. Very young and very old patients are at higher risk of developing disseminated disease, as are patients receiving immunosuppressants and those with HIV infection. Underlying medical conditions often associated with miliary tuberculosis include alcoholism, malignancy, connective tissue diseases, renal failure, and pregnancy. However, it must be emphasized that absence of an underlying disease does not exclude the possibility of miliary tuberculosis.
About Miliary Tuberculosis
- The disease develops in very young, very old, and HIV-infected patients.
- It is also associated with alcoholism, malignancy, connective tissue diseases, renal failure, and pregnancy.
- In children, it presents with high fever, night sweats, weight loss, hepatosplenomegaly, and lymphadenopathy.
- Adults usually show moderate- to low-grade fever, night sweats, malaise, anorexia, weakness, and weight loss.
- Look for choroid tubercles inthefundi (present in up to 50% of cases).
- Provokes leukemoid reaction, anemia, hyponatremia, abnormal liver function tests. May also produce adrenal insufficiency.
- Micronodular interstitial pattern on chest radiographs; may be negative in elderly and HIV-infected patients.
- Blood samples, transbronchial biopsy, bone marrow samples, and liver biopsy may all yield positive cultures.
- Provide early treatment for all suspected cases, using isoniazid, rifampin, ethambutol, and pyrazinamide.
Children usually present to the physician with high fever, night sweats, weight loss, hepatosplenomegaly, and lymphadenopathy. However in adults, particularly elderly people, the clinical manifestations may be subtle. Patients usually have nonspecific complaints of fever, malaise, anorexia, weakness, and weight loss. Night sweats are also common.
Physical exam usually reveals a chronically ill patient with no specific findings. In some patients, lymphadenopathy may be detected. In all patients, fundoscopic exam should be carefully performed following pupillary dilation and may reveal choroid tubercles in up to 50% of cases. The diagnosis is often missed, and in up to 20% cases, it is made postmortem.
The peripheral white blood cell count is usually normal; however, some patients develop extremely high white blood cell counts (30,000 to 40,000/mm3), also termed a “leukemoid reaction,” that can be mistaken for leukemia. Pancytopenia can also develop. Liver function abnormalities are common. Elevated alkaline phosphatase and moderate increases in transaminase values are found in most patients. Serum sodium may be low as a consequence of adrenal insufficiency (a well-known complication of miliary tuberculosis) or inappropriate antidiuretic hormone secretion. Morning and evening serum cortisol levels should be measured to exclude adrenal insufficiency. In approximately two thirds of patients, Chest X-Ray reveals small nodules (0.05 to 1 mm in diameter) that resemble millet seeds (the basis for the designation “miliary”); however, a negative Chest X-Ray in elderly patients and in patients with HIV does not exclude this diagnosis. In a few patients, adult respiratory distress syndrome may develop, causing complete opacification of the lungs.
The key to the diagnosis of miliary tuberculosis is a high index of suspicion. Sputum smears are positive in only a few patients. Samples from enlarged lymph nodes, liver biopsy, and bone marrow should therefore be sought for histopathology (seeking granulomas and acid-fast bacilli) and culture. Transbronchial biopsy can yield the diagnosis in many patients. Blood samples for culture should be drawn; in AIDS patients, cultures are commonly positive. If Central nervous system symptoms are noted, a lumbar puncture should also be performed, although the resulting smears are usually negative.
A delay in treatment can have fatal consequences. Therefore, if miliary tuberculosis is high on the differential diagnosis, empiric antituberculous therapy should be initiated as soon as samples for culture have been obtained. A 4-drug combination consisting of INH, rifampin, pyrazinamide, and ethambutol is the preferred regimen. Patients usually defervesce within 7 to 14 days.
Secondary Tuberculosis
Reactivation of tuberculosis after primary disease occurs in 10% to 15% of patients. In half of these cases the infection reactivates within 2 years of exposure. In past decades, reactivation occurred most commonly in elderly patients, but in the United States today, most secondary cases are now reported in middle-aged adults (30 to 50 years of age). Early in the course of reactivation, patients are often asymptomatic, and evidence of reactivation is found only on Chest X-Ray. However if the infection is not detected, symptoms slowly develop and worsen over several months. The gradual nature of symptom onset often causes patients to delay seeing a physician. The patient in case 4.3 has the typical symptoms of secondary tuberculosis: a progressively worsening cough with sputum production, low-grade fever, night sweats, fatigue, and weight loss. Symptoms that suggest more advanced disease are hemoptysis (indicating erosion of a tuberculous cavity into an arteriole) and pleuritic chest pain (suggesting pleural involvement and probable tuberculous pleural effusion).
Physical exam is often unrevealing, as observed in case 4.3. Despite the presence of extensive pulmonary disease, auscultation may be normal. Fine rales may be heard in the apices after a short cough and quick inspiration or after full expiration followed by a cough and rapid inspiration (post-tussive rales).
The hallmark of secondary pulmonary disease is the presence of apical cavitary lesions on Chest X-Ray. Lesions usually develop in posterior segments of the upper lobes just below the clavicle. Less frequently, infiltrates are noted in the apex of the lower lobe (usually obscured by the heart shadow). In addition to routine posterior-anterior and lateral chest films, an apical lordotic view is often helpful in visualizing upper lobe apical lesions. A chest computed tomography scan can be helpful for assessing the extent of disease and for defining the size of the cavities. Unlike conventional lung abscesses, tuberculous cavities rarely have air fluid levels.
About Secondary Tuberculosis
- Reactivation occurs in 10% to 15% of patients, half within 2 years of primary disease.
- Reactivation is most common in men 30 to 50 years of age.
- Apical infection is most common.The high oxygen content and reduced lymphatic flow favor M. tuberculosis survival in this region.
- Symptoms progress slowly over several months: worsening cough with sputum production, low-grade fever, night sweats, fatigue, and weight loss.
- Hemoptysis or pleuritic pain indicate severe disease.
- Physical exam usually produces minimal findings; post-tussive rales may be seen.
- Chest radiograph shows apical cavities (without fluid); order apical lordotic. A computed tomography scan is often helpful.
- Cavitary disease is highly infectious; cavities contain between 109and 1010 organisms. Isolate all patients. In HIV infection, the chest radiograph often does not show cavities. All pneumonias in patients with AIDS are considered to involve tuberculosis until proven otherwise.
In patients with AIDS, infiltrates may be in any region of the lung and may not cavitate. Any HIV-infected patient with a new pulmonary infiltrate should therefore be considered to have tuberculosis until proven otherwise. In fact, in some instances, HIV-infected patients with active respiratory tuberculosis may have a negative Chest X-Ray.
Individuals with cavitary disease are potentially highly infectious. Cavities may contain between 10 and 10 organisms. Patients should be placed in respiratory isolation while sputum acid-fast bacilli smears and cultures are obtained. The number of organisms seen on smear directly correlates with infectiousness — that is, the higher the number of organisms per microscopic field, the higher the likelihood of disease spread.
Diagnosis
The classic test for making the diagnosis of pulmonary tuberculosis is the Ziehl-Nielson acid-fast sputum smear. Morning sputum samples tend to have the highest yield. A single negative smear should not delude the clinician into a false sense of security. Three sputum smears are recommended, because in cavitary disease, the release of infectious droplets is intermittent. Only after three smears are negative should the patient be declared to be at low risk for spreading infection. Negative smears do not definitively exclude tuberculosis.
To be positive, the sputum smear must contain 10 organisms per milliter. A fluorochrome stain using auramine-rhodamine is more sensitive and allows sputum to be examined at low magnification (20x or 40x magnification) as compared with conventional acid-fast bacilli smears that must be examined at high magnification (100x). Sputum smear has only a 60% sensitivity as compared with sputum culture. The Polymerase chain reaction technique can effectively detect as few as 10 organisms in a clinical specimen. Two assays, one using mycobacteria RNA as its initial template, and the other using mycobacterial Deoxyribonucleic acid, are commercially available. Sensitivity and specificity are greater than 95% in smear-positive cases, and specificity in smear-negative cases is high. False negative and false positive results are common in less experienced laboratories, and nucleic acid amplification assays are recommended only to complement traditional methods. In patients on antituberculous therapy, Polymerase chain reaction cannot differentiate killed from actively growing organisms. Culture remains the most accurate method for diagnosing M. tuberculosis. In patients that fail to produce sputum, aspiration of the gastric contents in the morning before the patient arises from bed is useful for obtaining samples for culture. In patients with suspected disseminated disease, blood samples in which all cells are lysed to release intracellular mycobacteria should be collected. The bacterium grows at about l/20th the rate of more conventional bacteria, taking 3 to 6 weeks to grow on Lowenstein-Jensen medium. Living mycobacteria can be more quickly detected in blood, sputum, pleural fluid, or cerebrospinal fluid using the Bactec radiometric or fluorometric culture system, which is designed to detect mycobacteria metabolism within 9 to 16 days. Drug susceptibilities can also be reliably tested using this method.
About the Diagnosis of Tuberculosis
- Ziehl-Nielson acid-fast stain can detect 104 organisms per milliter with 60% sensitivity.
- Release of acid-fast bacilli from cavitary lesions is intermittent. To ensure low infectivity, three negative smears are needed.
- Culture remains the most sensitive and specific test.
- Mycobacterium tuberculosis grows at l/20th the rate of conventional bacteria.
- Automated techniques can detect bacteria within 9 to 16 days.
- Growth in conventional Lowenstein-Jensen medium takes 3 to 6 weeks.
- Polymerase chain reaction is available, but should be performed only by experienced laboratories.
Treatment
The principal strategies for treating mycobacteria differ somewhat from more conventional bacteria. Because mycobacteria are intracellular and grow very slowly, and because dormant tuberculous organisms found in necrotic cavitary lesions are difficult to kill, antituberculous therapy must be prolonged — a period of months.
Secondly, because the number of mycobacterial organisms in the host is usually high, the potential for selecting for resistant mycobacteria is high. To reduce this risk, treatment with two or more antimycobacterial medications is recommended. Generally, 1 in 10 organisms is resistant to INH. Cavitary lesions often contain between 10 and 10 organisms, assuring the survival and replication of resistant organisms. Administration of two drugs reduces the probability of selecting for a resistant organism because only 1 in 1012 organisms (106 X 106) would be expected to be resistant to both antimicrobial agents.
A third major consideration is advent of multidrug-resistant M. tuberculosis. These mycobacteria are resistant to isoniazid and rifampin, and they must be treated with three or more other antimycobacterial agents. In the early 1990s in the United States, multidrug-resistant M. tuberculosis was major concern; however, with improved infection control measures, the use of four drug regimens, and directly observed therapy, the incidence of multidrug-resistant M. tuberculosis has been reduced to less than 2%, and resistance to INH alone is approximately 8%.
Resistance is classified as either secondary or primary. Primary resistance is defined as infection with a resistant strain in a patient who has never received antituberculous drugs. When a resistant strain is cultured from a patient who were previously treated for drug-sensitive tuberculosis, the infection is said to be secondarily resistant. Secondary resistance is a major problem among homeless people, illicit drug users, and patients with AIDS.
Table. Antituberculous Medications: Half-Life, Dosing, Renal Dosing, and Cost
| Antituberculous agent (trade name) | Halflife (h) | Dose | Dose for reducedcreatinine clearance
(mL/min) |
| First line | |||
| Isoniazid (Tubzid,Nydrazid) | 0.5-4 2-5 | 300 mg PO or IM q24h | No change required No change required |
| Rifampin (Rifadin,Rimactane) | early 2 late | 600 mg PO q24h | No change required |
| Pyrazinamide | 10-16 | 15-30 mg/kg PO q24h, divided into 2-4 doses | |
| Ethambutol (Myambutol) | 3-4 | 15-25 mg/kg POq24h | 50-80:15 mg/kg q24h 10-50:15 mg/kg q24-36h <10:15 mg/kg q48h |
| Streptomycin | 2-5 | 1-2glMorlVq24h | 50-80:15 mg/kg q24-48h 10-50:15 mg/kg q72-96h < 10:7.5 mg/kg q72-96h |
| Second line | |||
| Ciprofloxacin (Cipro) | 4 | 750mgPOq12h | 10-50:q18h/<10:q24h |
| Amikacin (Amikin) | 2 | 7-10 mg/kg IM or IV q24h (not to exceed 1 g), 5 X/week | Renal dosingbased on serum levels |
| Capreomycin (Capastat) | 4-6 | 1 g IM q24h | 10-50:7.5 mg/kg q24-48h <10:7.5 mg/kg X 2 weekly |
| Cycloserine (Seromycin) | 8-12 | 250-500 mgPOq12h | 10-50:250-500 mg q24h <10:250mgq24h |
| Para-aminosalicylic acid | 2 | 10-12gPOq24h in 3-4 divided doses | Obtain from the U.S. Centersfor
Disease Control and Prevention |
| Ethionamide (Trecator) | 4 | 0.5-1 g PO q24h in 1 -3 doses | <10:5mg/kgq48h |
Table. Typical Course of Direct Observed Therapy for Tuberculosis
| Timing | Frequency | Regimen |
| Weeks 1-2 | Once daily | Isoniazid 300 mgRifampin 600 mg
Pyrazinamide 1.5 g (<50 kg),2 g (51-74 kg), 2.5 g (>74 kg) Streptomycin 750 mg (<50 kg) or 1 g (>50 kg) |
| Weeks 3-8 | Twice weekly | Isoniazid 15 mg/kgRifampin 600 mg
Pyrazinamide 3 g (<50 kg),3.5 g (51-74 kg),4.0 g (>74 kg) Streptomycin 1 g (<50 kg), 1.25g (51-74 kg), 1.5 g (>74 kg) |
| Weeks 9-26 | Twice weekly | Isoniazid 15 mg/kg Rifampin 600 mg |
Outside of the United States, the percentages of multidrug-resistant M. tuberculosis and INH-resistant strains vary widely. The worldwide median frequency of primary INH resistance is estimated to be 7.3%, with higher levels being observed in Asia, Africa, and Latin America, and lower levels in Europe and Oceania. The worldwide incidence of primary multidrug-resistant M. tuberculosis is 1.4%; however, rates of multidrug-resistant M. tuberculosis as high as 14% have been reported in countries inwhich tuberculosis control programs have deteriorated (Latvia, South Korea, and Russia, for example). Extensively drug resistant tuberculosis (XDR) has recently been reported in South Africa. XDR ТВ fails to respond to nearly all drugs. The mortality can exceed 90%.
The various antituberculous agents have been classified as first-line and second-line drugs. First-line medications include INH, rifampin, pyrazinamide, streptomycin, and ethambutol. These agents are more efficacious and less toxic than the second-line drugs. With the exception of ethambutol, first-line agents are also bactericidal. Whenever possible, first-line drugs should be employed for the treatment of M. tuberculosis. The recommended treatment for presumed drug-sensitive pulmonary tuberculosis (pending sensitivity tests) is a four-drug regimen: INH, rifampin, pyrazinamide, and ethambutol or streptomycin. This regimen is recommended for 2 months, to be followed by INH, rifampin, and pyrazinamide for 4 months. If multidrug-resistant M. tuberculosis is suspected, extensive susceptibility testing should be performed, and expert advice sought to design an appropriate regimen. Treatment should consist of at least three drugs to which the organism is proven to be susceptible. Fluoroquinolones combined with amino-glycosides are particularly useful for treating multidrug-resistant M. tuberculosis.
In patients who are deemed to be unreliable, directly observed therapy should be instituted. Poor adherence greatly increases the risk of secondary multidrug-resistant M. tuberculosis, and with the institution of directly observed therapy in these patients, the emergence of resistance is minimized. Directly observed therapy is recommended for all patients with INH- or rifampin-resistant organisms.
About Antieuberculous Therapy
- A four-drug regimen (pending sensitivity testing) is recommended.
- Of every 106 organisms, 1 is naturally resistant to one drug.
- Cavitary lesions contain between 109 and 1010 organisms.
- A minimum of two effective drugs are needed to prevent resistance (106 X 106 = 1012).
- Primary isoniazid (INH) resistance is common to reliably prevent resistance,treat with INH, rifampin, pyrazinamide,and ethambutol (pending sensitivities).
- INH-resistance is 8% in the United States, 7.3% worldwide. Higher in Asia, Africa, and Latin America.
- Multidrug resistance is below 2% in the United States, but up to 14% in parts of Eastern Europe.
- Secondary resistance occurs in patients who don’t reliably take their medications.
- Directly observed therapy is now recommended for unreliable patients,and for patients with INH- or rifampin-resistant strains.
Prevention
Tuberculosis is spread strictly from person to person. Identifying and preventing individuals who have been exposed to tuberculosis from developing active disease is a major public health goal. The purified protein derivative test is a very helpful skin test that assesses exposure to tuberculosis. The test is produced by acid precipitation of tubercle bacilli proteins, and the 5-tuberculin unit dose has been standardized and is administered as a 0.1-mL subcutaneous injection on the volar aspect of the forearm. Deeper injection is ineffective because tuberculous proteins can be removed by blood flow, producing a false negative result.
The injection should produce a discrete raised blanched wheal. The test is read 48 to 72 hours after injection; however, the reaction usually persists for 1 week. The diameter of induration is measured, and a diameter of more than 10 mm is defined as positive. A positive test indicates high risk for contracting tuberculosis. Of people with a purified protein derivative reaction 10 mm in diameter, 90% are infected with tuberculosis. If the reaction measures more than 15 mm, 100% are infected. The 15-mm diameter is defined as a positive reaction in individuals with no risk factors for tuberculosis. In individuals who are immunocompromised (HIV-positive, organ transplant patients receiving more than 15 mg prednisone daily) or who are recent household contacts of a patient with active tuberculosis, more than 5 mm is considered a positive reaction.
A positive test simply indicates that, sometime in the past, the individual was exposed to active tuberculosis; however, this finding does not indicate active disease.
About Tuberculosis Testing and Prophylaxis
- The purified protein derivative test is carefully standardized, and induration at 48 hours is considered positive at
- more than 5 mm in people who are HIV-positive or immunocompromised, or who have had recent household exposure;
- more than 10 mm in people at overall riskfor exposure; and
- more than 15 mm in people with no risk factors.
- A positive result indicates exposure sometime in the past; negative to positive conversion indicates exposure during the period between tests.
- Prophylaxis with isoniazid (300 mg daily for 6 months) if
- conversion within the last two years and negative chest radiograph (Chest X-Ray).
- positive purified protein derivative and negative Chest X-Ray (recommendation of the Centers for Disease Control and Prevention).
- abnormal Chest X-Ray, and three follow-up sputum smears for acid-fast bacilli are negative.
- If prophylaxis recipient is older than 35 years, a consumer of alcohol or other hepatotoxic drugs, pregnant, or HIV-positive, risk of INH hepatitis requires monthly monitoring of hepatic enzymes.
The conversion from negative to positive in an individual who is tested annually indicates exposure to tuberculosis during the time interval between tests. Tuberculin skin tests are useful in otherwise healthy individuals, but cannot be relied upon to determine exposure in HIV patients with low CD4 counts, in patients receiving immunosuppressants, or in patients with severe malnutrition.
Individuals with a positive purified protein derivative should have a Chest X-Ray, and if pulmonary lesions are noted, three sputum samples should be obtained for culture and smear. Prophylaxis should be given only if all sputum samples prove negative for tuberculosis. Because the risk of developing active disease is highest within 2 years of exposure, all individuals who have converted from a negative to a positive test within 2 years should receive INH prophylaxis. Preventive therapy is also warranted when a positive test is associated with other specific risk factors (HIV infection, known recent exposure to tuberculosis, abnormal Chest X-Ray, intravenous drug abuse, and certain underlying diseases).
In other individuals with a positive purified protein derivative, the risk of INH hepatotoxicity must be balanced against the likelihood of preventing the development of active disease.
The U.S. Centers for Disease Control and Prevention currently recommends that all individuals with a positive purified protein derivative receive prophylaxis. However, in individuals 35 years of age or older, the risk of hepatotoxicity may outweigh the potential benefit of INH prophylaxis. Hepatic enzymes should be monitored at monthly intervals in HIV-positive patients, pregnant women, patients with underlying liver disease, and in those receiving other potentially hepatotoxic drugs or drinking alcohol daily. Prophylaxis should discontinued if transaminase levels rise exceed 3 times the normal values in association with symptoms consistent with hepatitis.
The recommended prophylactic regimen is INH 300 mg daily for 6 months. For HIV-infected patients, 12 months of INH prophylaxis is recommended.
Atypical Mycobacteria
Atypical mycobacteria are found throughout the environment in soil and water. These organisms have a low virulence, and they do not usually cause pulmonary disease in otherwise healthy individuals. In patients with underlying pulmonary disease, these organisms can be inhaled and cause pulmonary infection.
M. avium complex is the most common of the atypical mycobacteria to infect the lung. A cavitary upper lobe lesion is the usual manifestation of this disease. The cavities tend to be somewhat smaller and thinner walled than those with M. tuberculosis. Pulmonary infection with M. avium complex is seen primarily in male smokers in their early fifties who abuse alcohol. Infection of the lungs is also seen in women 60 year of age or older with no apparent underlying disease, most commonly involving the right middle lobe or lingula.
About Atypical Mycobacteria Pulmonary Infection
- Atypical mycobacteria are found in soil and water.
- Infects males over the age of 50 years, who are also alcoholic, smokers with chronic lung disease. Often presents as upper lobe cavitary disease.
- Infects women over the age of 60 years without apparent underlying disease. Presents as right middle lobe or lingular disease.
- M. avium is the most common pathogen; M.kansasii,M.fortuitum, and M.abscessus are rarer.
- Management is complex and requires a pulmonary or infectious disease specialist.
M. kansasii, M. fortuitum, and M. abscessus can also infect the lungs, causing chronic cavitary disease. Because these organisms are found throughout the environment and may colonize as well as infect patients with chronic lung diseases, elaborate criteria for differentiating colonization from infection have been established. Therapy for atypical mycobacterial infection must be prolonged and is based on sensitivity testing. Often these organisms respond poorly to therapy, and resection of the infected lung segment may be required for cure. Management of these patients is complex and requires the supervision of an experienced pulmonary or infectious disease specialist.
Fungal Pneumonias
The most common forms of fungal pneumonia in the normal host are histoplasmosis and coccidiomycosis. In the immunocompromised host, Cryptococcus and Aspergillus can also cause pneumonia.
Histoplasmosis
Epidemiology
Histoplasma capsulatum is one of the more common causes of chronic pneumonia in the Midwestern and Southeastern United States. This organism survives in moist soil in temperate climates and is most commonly reported in the Ohio and Mississippi River valleys. The development of histoplasmosis is generally associated with construction or excavation of soil contaminated with H. capsulatum. Infection is also reported in spelunkers, who contract the infection by disturbing dried bat guano containing high concentrations of infectious particles. Exposure to infectious particles can also occur after the renovation of old buildings previously inhabited by birds or bats.
Pathogenesis
H. capsulatum is a fungus and exists in two forms: mycelia or yeast. In the moist soil of temperate climates, the organism exists in the mycelial form as macroconidia (8 to 15 um in size) and microconidia (2 to 5 um in size). When infected soil is disturbed, microconidia float in the air and can be inhaled into the lung. Once in the lung, microconidia are ingested by alveolar macrophages and neutrophils. In the intracellular environment of these phagocytes, the mycelia transform to rounded, encapsulated yeast cells. During this transformation, multiple genes are upregulated, including a gene that increases production of a calcium-binding protein important for acquiring calcium (an essential ion for yeast survival) from the intracellular environment. The expression of this calcium-binding protein may explain the frequent finding of calcifications in infected tissues.
About the Epidemiology and Pathogenesis of Histoplasmosis
- Found primarily in the Midwest and Southeast United States.
- Grows in moist soil in temperate zones, mainly Ohio and Mississippi River valleys.
- Found in caves and old buildings; bat guano is a concentrated source.
- Mycelial form in soil, as macro- and microconidia. Microconidia readily aerosolized.
- Inhaled microconidia ingested by macrophages and neutrophils convert to yeast forms and upregulate many genes, including a gene for calcium binding.
- Yeast forms are transported to hilar nodes, where cell-mediated immunity is induced.
As is observed in tuberculosis, infected macrophages transport the yeast forms to the hilar lymph nodes where Histoplasma antigens are presented to T cells. Within several weeks, cell-mediated immunity develops, and CD4 T cells activate macrophages to produce fungicidal products.
Clinical Manifestations
In more than 90% of patients, infection is controlled. In many patients, primary exposure is asymptomatic or results in a mild influenza-like illness. Very young people, elderly people, and patients with compromised immune systems are more likely to develop active disease. Symptoms usually develop within 14 days of exposure and may include high fever, headache, nonproductive cough, and dull nonpleuritic chest pain. This form of chest pain is thought to be the result of mediastinal node enlargement. In other patients, chest pain may be sharper and may worsen upon lying down, reflecting the development of pericarditis (observed in approximately 6% of cases).
On Chest X-Ray, patchy infiltrates may be seen during acute disease that subsequently calcify producing a “buckshot” appearance. Healed histoplasmosis is also the most common cause of calcified lesions in the liver and spleen. In acute disease, mediastinal lymphadenopathy may be prominent and may mimic lymphoma or sarcoidosis. A history of exposure to a site where soil was excavated is particularly important in trying to differentiate between these various possibilities.
About the Clinical Manifestations of Histoplasmosis
- In 90% of cases,a brief self-limiting flu-like illness occurs or the person remains asymptomatic.
- Disease can develop in elderly, very young, and immunocompromised individuals.
- At 14 days post exposure,the individual may have
- high fever, headache, nonproductive cough, and dull, nonpleuritic chest pain.
- a Chest X-Ray with patchy infiltrates that later convert to “buckshot” calcifications.
- mediastinal lymphadenopathy that may mimic lymphoma or sarcoidosis.
- Progressive mediastinal fibrosis (a rare complication).
- Cavitary disease is clinically similar, with men older than 50 years who have chronic obstructive pulmonary disease at higher risk.
- Disseminated disease occurs in 10% of symptomatic primary disease.
- Likelihood of dissemination occurs in people who are very old, very young, or immunosup-pressed (because of AIDS or transplantation).
- Meningitis with lymphocytosis and low glucose may develop.
- Reticulonodular pattern on Chest X-Ray in most cases, but Chest X-Ray normal in one third.
Occasionally, mediastinal nodes can become massively enlarged, reaching diameters of 8 to 10 cm. Severe mediastinal fibrosis is rare, but it can lead to impingement and obstruction of the superior vena cava, bronchi, and esophagus.
Chronic cavitary histoplasmosis develops in about 8% of patients. This complication is more common in men over the age of 50 years who have chronic obstructive pulmonary disease. The symptoms and Chest X-Ray findings associated with chronic pulmonary histoplasmosis are indistinguishable from cavitary tuberculosis. In fact, in the past, patients in the Midwestern and Southeastern United States with chronic pulmonary histoplasmosis were frequently misdiagnosed as having pulmonary tuberculosis and were mistakenly confined to tuberculosis sanatoriums. Spontaneous resolution of cavitary disease occurs in 10% to 60% of cases.
Progressive disseminate histoplasmosis occurs in about 10% of symptomatic primary infections. Progressive dissemination also develops as a consequence of reactivation of old disease. In the immunosuppressed individual, reactivation is the most likely pathway for disseminated disease. Onset of symptoms is usually abrupt. Fever and malaise are followed by nonproductive cough, weight loss, and diarrhea. Hepato-splenomegaly usually develops, and lymphadenopathy may be detected. Anemia, thrombocytopenia, and leukopenia are observed in a high proportion of patients. Meningitis may develop, resulting in lymphocytosis and low glucose in the cerebrospinal fluid. A Chest X-Ray may show a reticulonodular pattern or scattered nodular opacities; however, the Chest X-Ray is normal in nearly one third of cases. Mortality is high if treatment is not initiated.
Diagnosis
H. capsulatum can be readily grown from tissue samples and body fluids using brain-heart infusion media containing antibiotics and cycloheximide (inhibits the growth of saprophytic fungi). Mycelial growth can usually be detected within 7 days and confirmed using a Deoxyribonucleic acid probe. The clinical microbiology lab must be notified that H. capsulatum is the possible pathogen, because the necessary culture methods are not employed on routine samples.
About the Diagnosis of Histoplasmosis
- Sputum culture is often positive.
- Requires selective media (brain-heart infusion with antibiotics and cycloheximide).
- Not a routine method; clinical microbiology must be notified.
- Bronchoscopy improves yield (90% in HIV patients).
- Bone marrow positive in 50% of cases.
- Lysis-centrifugation method positive in up to 50% of blood samples.
- Polysaccharide urine and serum antigen test is the most sensitive, being positive for
- 90% of disseminated disease,
- 40% cavitary disease,and
- 20% acute pulmonary disease
- Method can also be used to test bronchoscopic lavage fluid.
- Histopathology shows noncaseating or caseat-ing granulomas. Silver stain best for identifying the yeast forms. Hematoxylin-eosin is not useful; periodic acid Schiff may help with identification.
- Urine antigen test positive in 90% of disseminated histoplasmosis
About the Treatment of Histoplasmosis
- Itraconazole the oral agent of choice. Recommended for
- acute pulmonary disease that fails to improve over 7 days.
- extensive mediastinal involvement.
- progressive cavitary disease.
- Amphotericin В is used for more severe disease. Recommended for
- primary pulmonary disease when the patient cannot take oral medications.
- cavitary disease that fails to improve on itraconazole.
- progressive disseminated disease.
A single sputum culture has only a 10% to 15% yield; collection of multiple sputum cultures increases the yield. Bronchoscopy has proved useful for providing good sputum samples yielding positive cultures in 90% of HIV patients with pulmonary histoplasmosis. Bone marrow and blood cultures should also be obtained and are positive in up to 50% of cases. The lysis-centrifugation blood culture technique (also used to culture mycobacteria) is the most sensitive method. The most effective method for detecting progressive disseminated histoplasmosis is the urine and serum polysaccharide antigen test. Antigen is detected in up to 90% of patients with disseminated disease. The antigen test is also positive in 40% of patients with cavitary pulmonary diseases and 20% with acute pulmonary histoplasmosis. Pulmonary lavage fluid can also be tested in this manner. A Polymerase chain reaction method is available only on an experimental basis.
Histopathologic examination of infected tissue also allows for rapid diagnosis. Noncaseating or caseating granulomas may be seen. An excessive fibrotic reaction may be seen in some patients. Silver stains are most effective for identifying the typical yeast forms in tissue biopsies. Organisms are poorly visualized by hematoxylineosin staining, but can often be seen on periodic acid Schiff stain.
Treatment
Itraconazole is the most effective azole for oral treatment. In patients with acute pulmonary histoplasmosis who fail to improve over the first week, itraconazole 200 mg daily for 4 to 6 weeks is recommended. If the patient is unable to tolerate azoles or cannot take oral medications, amphotericin В 0.4 to 0.5 g/kg can be administered intravenously until symptoms subside. In patients with extensive mediastinal involvement, itraconazole 200 mg daily, can be given for 3 to 6 months. If rapid resolution of symptoms is necessary, amphotericin В is preferred.
Patients with severe mediastinal fibrosis may also require surgical intervention to correct vascular and airway obstruction. In cavitary pulmonary disease, progression of lesions over 2 to 3 months or persistent cavities associated with declining respiratory function warrant treatment with itraconazole 200 mg twice daily for a minimum of 6 months. Amphotericin В may be required if lesions fail to improve on itraconazole therapy. In acute, life-threatening progressive disseminated histoplasmosis, amphotericin В should be given in high doses: 0.7 to 1 mg/kg daily. Once the patient has defervesced, the dosage can be lowered to 0.4 to 0.5 mg/kg, or the patient can be switched to itraconazole 200 mg twice daily.
Coccidiomycosis
Epidemiology — Like H. capsulatum, Coccidioides immi-tis survives and grows in soil. The ideal conditions for survival of C. immitissxc dry, alkaline soil, hot summers, and winters with few freezes. These conditions exist in central California’s San Joaquin Valley and in the southern regions of Arizona, New Mexico, and Texas. С immitis is also found in Mexico, Central America, and South America. Infections are most commonly reported in the summer months when dry soil more readily forms dust particles. Epidemics have been associated with disruption of soil by archeological excavation, earthquakes, and dust storms. In recent years, the incidence of coccidiomycosis has increased as a consequence of the increased numbers of people living in endemic areas.
About the Epidemiology and Pathogenesis of Coccidiomycosis
- Grows in soil; prefers dry, alkaline soil, hot summers, and winters with few freezes.
- Primarily found in central California, southern Arizona, New Mexico, and Texas. Also found in Mexico, Central America,and South America.
- Contracted during the summer, often through dust storms,excavation, earthquakes.
- Mycelial form of this dimorphic fungus is called arthroconidia.
- Inhaled arthroconidia transform to spherules (yeast forms) that release endospores.
- Endospores ingested by macrophages are transported to hilar lymph nodes, lymphatics, and bloodstream.
- Cell-mediated immunity critical for control of infection.
Pathogenesis
Also like H. capsulatum, C. immitis is a dimorphic fungus. It exists in soil as mycelia that can form small arthroconidia (5-µm barrel-shaped structures). Arthroconidia can become airborne, whereupon they are inhaled by humans and become lodged in the terminal bronchioles. In the warm moist environment of the lung, the arthroconidia transform into spherules. As the spherules mature, their outer walls thin, and they release endospores that are ingested by macrophages. As is observed in histoplasmosis and tuberculosis, macrophages transport the infectious particles to the hilar lymph nodes, the lymphatic system, and the bloodstream, resulting in dissemination. Cell-mediated immunity is critical for control of the infection.
Clinical Manifestations
Approximately two thirds of patients exposed to arthroconidia experience minimal symptoms. When symptoms are noted, they usually develop 7 to 21 days after exposure. Nonproductive cough and fever are the most frequent symptoms. Pleuritic chest pain, shortness of breath, headache, and fatigue are also commonly reported. Skin manifestations may include erythema nodosum (red, painful nodules on the anterior shins), erythema multiforme (target-like lesions involving the entire body, including the palms and soles) or a nonpruritic papular rash. Arthralgias may develop in association with erythema nodosum. Eosinophilia is commonly observed on peripheral blood smear.
In about half of patients, a Chest X-Ray is abnormal, most commonly demonstrating unilateral infiltrates, pleural effusions, and hilar adenopathy. In patients with depressed cell-mediated immunity (primarily patients with AIDS and CD4 counts below 100/mm3), the infection can disseminate, causing diffuse opacification of the lungs and severe respiratory failure. Meningitis, skin lesions, bone infection and arthritis may also develop as a consequence of dissemination.
In some patients pulmonary infection can persist, causing progressive destruction of lung parenchyma associated with a productive cough, chest pain, weight loss. A Chest X-Ray may demonstrate areas of fibrosis, nodules, cavitary lesions, or a combination. An isolated nodule can persist in approximately 4% of pulmonary cases and can be differentiated from neoplasm only by biopsy. These lesions seldom calcify as the lesions of histoplasmosis do. A chronic pleural effusion can result from the rupture of a peripheral cavitary lesion into the pleural space. This complication is most commonly reported in young, otherwise healthy, athletic males.
About the Clinical Manifestations of Coccidiomycosis
- Symptoms (nonproductive cough, fever, pleuritic chest pain, shortness of breath, headache, and fatigue) occur in about one third of exposed individuals 7 to 21 days after inhalation.
- Skin manifestations are common: erythema nodosum, erythema multiforme, nonpruritic papular rash.
- Eosinophilia may be noted on peripheral blood smear.
- Abnormal Chest X-Ray findings are frequent: unilateral infiltrates, pleural effusions, hilar adenopathy.
- In patients with AIDS whose CD4 counts fall below 100/mm3, the disease can disseminate, causing diffuse lung opacification, meningitis, bone infection, and arthritis.
- Chronic lung disease can lead to fibrosis, nodules, or cavities.
- Isolated pulmonary nodules are not calcified, can be differentiated from neoplasm by biopsy.
- Chronic pleural effusions most commonly develop in young, healthy,athletic males.
Diagnosis
Travel to, or past residence in, an endemic area should alert the clinician to the possibility of coccidiomycosis. Examination of induced sputum or sputum obtained by bronchoscopy may reveal spherules. The fungus is not seen on Gram stain, but can be detected by silver stain. Biopsies of infected tissue should be obtained; they usually reveal caseating or noncaseating granulomas and spherules. The organism grows readily as a white mold on routine mycology media and on bacterial media under aerobic conditions.
Multiple serologic tests are available. These tests are often required to make the diagnosis, because of unavailability of sputum and biopsy specimens. Immunoglobulin M serum titers against С immitis are usually positive within the first week of disease. Immunoglobulin G levels are most commonly tested by complement fixation or immunodiffusion. Levels of Immunoglobulin G increase after Immunoglobulin M and often persist for years. A correlation has been observed between the Immunoglobulin G serum titer and severity of disease. A rising titer that exceeds 1:32 may signal disseminated disease; a falling titer indicates a favorable prognosis. Patients with no detectable lesions can have titers below 1:8 for many years after exposure.
About the Diagnosis of Coccidiomycosis
- Spherules may be seen on induced sputum or after bronchoscopy.
- The organisms are readily cultured on routine bacterial and mycology culture plates.
- Histopathology shows noncaseating and caseating granulomas;Gram stain is not useful, silver stain is best.
- Multiple serology tests are available to measure immunoglobulin G and M antibody titers.
- The Immunoglobulin M titer is elevated in acute disease.
- The Immunoglobulin G level often persists for years. A rising titer exceeding 1:32 signals dissemination; a falling titer is indicative of a favorable prognosis.
Treatment
Most infections with this organism spontaneously resolve. Treatment is reserved for patients with disseminated disease and patients with persistent or progressive coccidioidal pneumonia with hypoxia. Treatment needs to also be considered in patients with pulmonary disease who are at increased risk for dissemination, including people that are black, Philippino, pregnant, diabetic, and immunosuppressed (including patients with AIDS).
Amphotericin В remains the preferred initial therapy for life-threatening disseminated disease or severe pulmonary disease until the infection is under control. High doses of amphotericin В (0.7 to 1 mg/kg daily) are recommended. In less severe disease, fluconazole (400 to 800 mg daily) or itraconazole (200 mg twice daily) are used. These agents are preferred because of their low toxicity and suitability for prolonged therapy. Treatment should be continued until symptoms and signs of infection have resolved. A minimum of 6 months’ therapy is recommended. In patients with meningeal involvement, triazole therapy should be continued indefinitely.
Surgical debridement of large purulent collections is recommended. Resection of rapidly expanding pulmonary cavities should be performed to prevent rupture into the pleural space. Surgical resection is also recommended to prevent bronchopleural fistula formation and to correct life-threatening pulmonary hemorrhage.
About the Treatment of Coccidiomycosis
- Treatment usually reserved for disseminated disease.
- Amphotericin В given for severe disease.
- Fluconazole or itraconazole given for less-severe disease.
- Treatment continues for a minimum of б months.
- Complement fixation immunoglobulin G titers should decline to a stable low tier.
- For meningitis, triazole therapy should be continued indefinitely.
- Surgical resection can be used to expand lung lesions.