Essentials of Diagnosis
- Key signs and symptoms include dermal lesion (bacillary angiomatosis and chronic phase of Bartonella bacilliformis infection); papule at inoculation site followed by proximal lymphadenopathy (cat scratch disease); fever, bacteremia, acute hemolytic anemia (acute phase of B bacilliformis infection); persistent or relapsing fever (fever and bacteremia/endocarditis).
- Predisposing factors include louse exposure, low income, and homelessness (Bartonella quintana-associated bacillary angiomatosis, fever, and bacteremia/endocarditis); cat exposure (cat scratch disease and Bartonella henselae-associated bacillary angiomatosis, fever and bacteremia/endocarditis); sandfly exposure in endemic areas of South American Andes (B bacilliformis infection).
- History of HIV or immunocompromise (bacillary angiomatosis).
- Key laboratory findings include small, curved, pleomorphic weakly gram-negative bacilli, best visualized with Warthin-Starry silver stain of tissue.
General Considerations
There are currently 11 known species of Bartonella, four of which are considered to be pathogenic in humans, namely B bacilliformis, B quintana, B henselae, and Bartonella elizabethae. B henselae and B elizabethae have only recently been isolated and identified, but B quintana and B bacilliformis have long been known as the causes of trench fever (B quintana) and Oroya fever and verruga peruana (B bacilliformis). The bartonellae establish intimate relationships with animal hosts, often within the vascular compartment but without causing disease.
The relationship between B bacilliformis and the other three Bartonella species that are pathogenic in humans was established in the early 1990s. This followed the recognition of a new clinical syndrome, bacillary angiomatosis in HIV-infected individuals, in 1983. B henselae was first identified with molecular methods as a cause of this syndrome in 1990, and both B henselae and B quintana were cultured from tissue samples of patients with bacillary angiomatosis in 1992. B henselae was also associated with cat scratch disease in 1992. B bacilliformis is closely related to B henselae, B quintana, and B elizabethae, as indicated by 16S ribosomal RNA gene sequence analysis. Therefore, these last three organisms, formerly designated as Rochalimaea species, have been reclassified as members of the genus Bartonella. The spectrum of human disease associated with the bartonellae includes regional and disseminated granulomatous disease in immunocompetent hosts (cat scratch disease), persistent bacteremia or endocarditis, and vascular proliferative disease (verruga peruana or bacillary angiomatosis) in immunocompromised hosts (Box 1). B henselae and B quintana are the recognized causes of bacillary angiomatosis, while only B henselae has been implicated as a cause of cat scratch disease. Finally, these two species along with B elizabethae have all been identified in patients with endocarditis and bacteremia.
A. Epidemiology. B quintana is globally endemic. However, epidemics do occur. These epidemics are associated with conditions of poor sanitation and personal hygiene as seen in battlefield troops in World War I. Recent reports have shown a reemergence of trench fever among chronic alcoholics and the homeless in urban areas of the United States and western Europe. Urban trench fever is sporadic, both geographically and temporally, for unclear reasons. The human body louse, Pediculus humanus, is the only known vector of B quintana. B henselae is also globally endemic.
Cat contact appears to be the single most important factor in the development of cat scratch disease and B henselae-associated bacillary angiomatosis, which reflects the fact that cats are the most important reservoir for B henselae.
There are ~ 22,000 cases of cat scratch disease reported per year in the United States, making this the most common Bartonella-associated disease. Over 80% of cases occur in individuals < 21 years of age. The number of cases peaks in the fall and winter months. This seasonality may reflect differences in human behavior that place persons at risk of exposure to Bartonella species, or it may reflect breeding patterns of cats and fleas.
Approximately 18% of household members with cats are seropositive for B henselae antibodies. Risk factors for the development of cat scratch disease include ownership of a kitten (especially one with fleas) and being scratched or bitten by a kitten. Cats in the southeastern United States, coastal California, the Pacific Northwest, and the south central plains have the highest prevalence of B henselae antibodies (36-81%). These areas of high prevalence are regions of greater warmth and humidity and closely correlate with the distribution in the United States of the cat flea, Ctenocephalides felis. The lack of B henselae antibody in cats is highly predictive of the absence of bacteremia.
Approximately 40% of cats are bacteremic with B henselae; kittens are more likely to be bacteremic than adult cats, as are cats that spend most of their time outdoors. B henselae has also been isolated from fleas of bacteremic cats.
The cat flea has been shown to be a competent vector in the transmission of B henselae among cats. In the absence of the cat flea, direct cat-to-cat transmission among kittens has not been demonstrated. The role of the flea in transmission of B henselae to humans has not been elucidated. Recently, an association between another Bartonella species, Bartonella clarridgeiae, and cat scratch disease was reported after isolation of this organism from the blood of a flea-infested kitten that induced cat scratch disease in a human.
However, the role of B clarridgeiae as a cause of human infection is unclear. Although > 90% of patients with cat scratch disease report recent cat contact, only two-thirds of patients with bacillary angiomatosis report recent cat exposure, suggesting that there are other risk factors for developing bacillary angiomatosis. Both B henselae and B quintana are known causes of bacillary angiomatosis, and yet the risk factors and reservoirs for infection with these organisms are quite different. For B quintana-associated disease, risk factors include low income, homelessness, and exposure to lice.
Risk factors for B henselae-associated bacillary angiomatosis are similar to those for cat scratch disease, including cat ownership and being scratched or bitten by a cat. B henselae has also been found in fleas from cats owned by patients with bacillary angiomatosis. Despite the advent of serologic testing for Bartonella species, the incidence of bacillary angiomatosis remains unknown.
Of all reported cases of bacillary angiomatosis, ~ 90% are men that are coinfected with HIV. Cases have been reported from all areas of the United States, with greater numbers in New York City, San Francisco, and some parts of Florida and Texas. Bacillary angiomatosis is uncommon in immunocompetent individuals; conversely, it can occur in patients who are immunosuppressed from causes other than HIV. Bartonella bacteremia with fever occurs in both immunosuppressed and immunocompetent individuals. Because special, nonroutine techniques are required for its isolation, bacteremia with Bartonella spp. is likely to be underreported. B bacilliformis is geographically confined to intermediate altitudes (between 1000 and 3000 m) of the South American Andes. This coincides with the limited distribution of its sandfly vector, Lutzomyia vernicarum. B. Microbiology. Bartonella species are small, slightly curved gram-negative rods measuring 2um by 0.6 um. They are classified within the alpha subdivision of the division Proteobacteria, as are the Rickettsia, Ehrlichia, and Brucella.
They are oxidase-negative, aerobic, and fastidious in their laboratory growth requirements. B henselae and, to a lesser extent, B quintana, display twitching motility when mounted in saline, due to the presence of pili. B bacilliformis has polar flagella. Bartonella species can be cultivated when plated on freshly prepared, enriched (blood-containing) solid media and placed in a humidified atmosphere containing 5% CO2. Optimum temperature for growth is 35-37 °C, except for B bacilliformis, which grows best at 25-30 °C. Colonies are detected in 5-21 days.
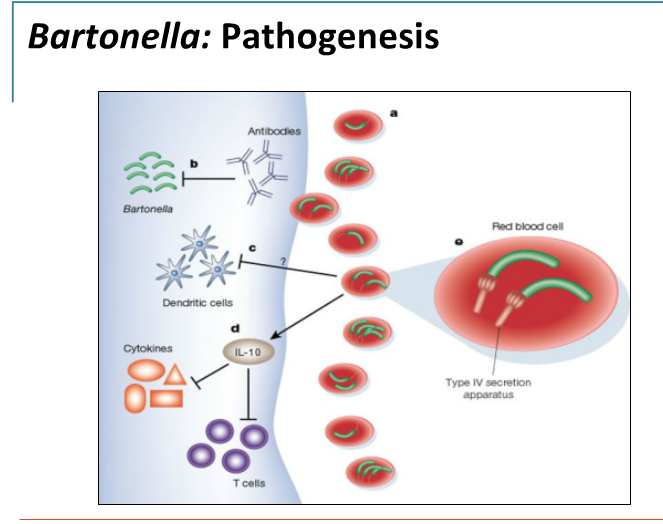
The colonies of Bartonella species from primary isolation are either white, raised, rough, and autoadherent or small, tan, moist appearing, and imbedded in the agar. Both types of colonies are usually seen in the same culture, but B henselae is usually more heterogeneous with a larger proportion of the rough morphology than B quintana, which has predominantly smooth colonies. B henselae and B quintana are difficult to distinguish because they are closely related at both the phenotypic and genotypic levels. C. Pathogenesis. Pili are believed to be key factors in host cell attachment as well as important virulence factors for B henselae and B quintana. The pathologic features of cat scratch disease and bacillary angiomatosis are quite different.
The hallmark of cat scratch disease is granuloma formation, with progression from lymphoid hyperplasia through granuloma formation to suppuration. Involved lymph nodes demonstrate a mixture of nonspecific inflammatory reactions including granulomata and stellate necrosis with lymphocytic infiltrates and multinucleated giant cells. Bacillary angiomatosis is a result of lobular proliferation of blood vessels. Small capillaries are arranged around ectatic ones with edema, mucin, or fibrotic stroma surrounding the lobules.
The endothelial cells that make up the vascular lobules occur either singly, in small groups, or in areas of solid proliferation. Endothelial cell necrosis may also be seen along with neutrophils and sometimes macrophages. In most cases, clusters of neutrophils and neutrophilic debris are seen adjacent to the capillaries scattered throughout the lesion. It is believed that B henselae and B quintana directly stimulate the vascular proliferation seen in the lesions of bacillary angiomatosis.
Angioproliferative lesions, however, have not been reported in patients with other forms of B henselae and B quintana infection such as trench fever and cat scratch disease. The flagella of B bacilliformis allow invasion of human erythrocytes, and the resulting cell lysis leads to the severe hemolytic anemia seen in the early stages of South American bartonellosis (Oroya fever). The nodular skin lesions that develop in the late stages of this disease reveal a characteristic vascular proliferation of endothelial cells that resembles bacillary angiomatosis.
CLINICAL SYNDROMES
CAT SCRATCH DISEASE
Clinical Findings
A. Signs and Symptoms. Cat scratch disease typically begins with an erythematous (“inoculation”) papule 3-5 days after cat contact or scratch (Table 1). The papule may appear on the skin, conjunctiva, or other mucous membrane. Lymphadenopathy follows 1-7 weeks later in a proximal distribution with the lymph nodes of the axilla, cervical, and submandibular areas most commonly involved. The lymph node is often tender, markedly swollen, and may ultimately suppurate. In 75% of all cases, the illness is mild, with myalgias, malaise, anorexia, and, rarely, nausea and abdominal pain.
Fever is present in about one-third of patients; temperatures above 39 °C occur in < 10%. Cat scratch disease is typically a benign and self-limited illness that lasts 6-12 weeks. Most cases of cat scratch disease occur in individuals with normal immune systems, but several cases have been described in immunocompromised patients.
B. Differential Diagnosis. The differential diagnosis of cat scratch disease includes many infections known to cause lymphadenopathy: atypical mycobacterial infection, tuberculosis, streptococcal and staphylococcal adenitis, toxoplasmosis, infectious mononucleosis, tularemia, histoplasmosis, and brucellosis. C. Complications. In as many as 14% of patients, dissemination or other complications occur. Parinaud’s oculoglandular syndrome is the result of conjunctival inoculation and is characterized by granulomatous conjunctivitis and preauricular adenopathy. It occurs in ~ 2% of all patients with cat scratch disease and usually resolves without sequelae. Hepatosplenic granulomata, lytic bone lesions, pneumonitis, and central nervous system involvement with encephalopathy and neuroretinitis can also occur. Symptoms of encephalopathy usually occur 1-6 weeks after the initial symptoms of cat scratch disease. Patients may become acutely confused and disoriented, rapidly progressing to coma, but they usually recover completely within 4 weeks. Neuroretinitis may be associated with a decrease in visual acuity, but marked improvement has been noted both with and without antibiotic treatment.
B BACILLIFORMIS-ASSOCIATED DISEASE
About 3 weeks after the bite of an infected sandfly, acute B bacilliformis infection becomes manifest as a febrile bacteremic illness (Oroya fever). It is accompanied by an acute hemolytic anemia that is caused by bacterial attachment and invasion of erythrocytes (Table 1). Patients exhibit malaise, fever, chills, diaphoresis, headache, and mental status changes. Lymphadenopathy, hepatosplenomegaly, and thrombocytopenia are also seen. At the end of the acute bacteremic phase, there is a transient immunosuppression that renders the patient susceptible to a wide range of opportunistic bacterial, viral, and parasitic secondary infections. Mortality during this acute phase is higher among persons without previous exposure to this agent. Overall, mortality is estimated to be ~ 8%. After the acute phase of infection, 15% of patients develop a chronic form of disease manifested by painless superficial or subcutaneous nodules (verruga peruana). These nodules are histologically similar to those of bacillary angiomatosis.
Fever & Bacteremia/Trench Fever/Endocarditis
Table 1. Clinical Manifestations of Bartonella Infections
Bacillary Angiomatosis
- Neovascular proliferative lesion, primarily of skin
- Liver and spleen involvement leads to bacillary peliosis
- May affect mucous membranes, lung, bone, and CNS
B bacilliformis Infection
- Acute phase: febrile bacteremic illness (Oroya fever) associated with acute hemolytic anemia
- Chronic phase: subcutaneous nodules (verruga peruana) resemble bacillary angiomatosis
Cat Scratch Disease
- Erythematous papule 3-5 days after cat contact at inoculation site
- Proximal lymphadenopathy usually of head, neck, or upper extremity
- Lymph nodes may suppurate
Fever and Bacteremia
- Often relapsing or persistent
- Endocarditis is sometimes associated
- Trench fever associated with B. quintana bacteremia
BOX 1. Bartonella Infection Syndromes
Children Adults More Common Cat scratch disease
- Bacillary angiomatosis
- Persistent bacteremia and/or endocarditis
Less Common
- Bacillary angiomatosis
- Persistent bacteremia and/or endocarditis
- B bacilliformis-associated disease
- B bacilliformis-associated disease
- Cat scratch disease
BOX 2. Treatment of Bartonella Infections
First Choice Second Choice Cat Scratch Disease Symptomatic therapy (resolves spontaneously) Azithromycin, 500 mg PO once on the first day, and 250 mg once daily for four subsequent days Bacillary Angiomatosis Erythromycin, 500 mg PO 4 times a day for at least 8 wksa Doxycycline, 100 mg PO twice daily for at least 8 wks1 B bacilliformis Infection Chloramphenicol (recommended in endemic regions) Fever and Bacteremia Erythromycin, 500 mg PO 4 times a day for at least 4 wks1 1Longer courses required if patient has bacillary peliosis, endocarditis, or relapses.
BOX 3. Control of Bartonella Infections
Prophylactic Measures
- Wash hands after animal contact; keep wounds and abrasions clean
- Avoid cats less than one year of age or minimize rough play
- Keep cats as free from fleas as possible
- Regular changing and washing of clothes and bedding
- Insecticides such as permethrin to control louse infestation
- Avoid the bite of the sandfly
Isolation Precautions
Useful articles
https://www.lymedisease.org/lyme-basics/co-infections/bartonella/
http://www.tiredoflyme.com/bartonella-symptoms.html
https://rawlsmd.com/health-articles/understanding-bartonella