Many viruses causing infection of the central nervous system (CNS) are covered in chapters devoted to each type of virus. For example, enteroviruses, the agents most frequently causing meningitis and occasionally encephalitis, are covered in site. The herpes viruses that cause meningitis, encephalitis, or both, especially herpes simplex virus (HSV), varicella-zoster virus (VZV), and Epstein-Barr virus (EBV), are discussed in site. This SITEconsiders viruses that cause CNS diseases (Box 1) as their primary manifestations.
Arthropod-Borne Viral Encephalitis
LYMPHOCYTIC CHORIOMENINGITIS (LCM)
Lymphocytic choriomeningitis (LCM) virus is an arenavirus, the same family as Lassa virus. All arenaviruses have a common reservoir in animals, especially small rodents. The infected animals may be asymptomatic or minimally diseased but excreting the virus in their secretions.

Epidemiology
LCM virus infects hamsters and mice; chronic infection is common in these animals and leads to chronic viremia and virus shedding in saliva, urine, and feces. Infection of humans may occur by way of aerosols, contamination of food, or fomites. Bites are not a usual mechanism of spread. Persistently infected rodents do not usually exhibit illness. The incubation period for LCM infections averages 10-14 days.
Microbiology
Arenaviruses are pleomorphic and enveloped with lipid; the virion has a mean diameter of 120 nm. They contain two-stranded RNA in a linear or circular configuration. The total molecular weight of this RNA is 3.2 × 106 daltons-4.8 × 106 daltons.
Pathogenesis
Arenaviruses are able to infect macrophages and possibly cause the release of mediators of cell and vascular damage. In certain laboratory animals the clinical severity of arenavirus disease appears to be directly related to the host’s immunologic response. The greater the immune (especially T lymphocyte) response, the worse the disease. Whether these mechanisms are operative in human infection is not clear. LCM virus may actually produce an encephalitis as well as meningitis. Perivascular mononuclear infiltrates may be seen in neurons of all sections of brain as well as in the meninges.
Clinical Syndrome
LCM illness occurs in most infected individuals but is usually nonspecific or influenzalike. Current estimates show that ~35% of infected persons exhibit clinical evidence of CNS infection. The name of the virus suggests that meningitis is a typical clinical event, but actually a febrile illness, if it occurs, may be subacute and persist for several months. Encephalitis occurs in approximately one-third of patients with CNS manifestations.
Laboratory Diagnosis
The diagnosis of LCM virus infection is usually made through serologic tests, although the virus can be recovered by inoculation of blood (early) or CSF (late in illness) into suckling mice or Vero monkey cells.
Treatment & Prevention
Only supportive therapy for patients with LCM infection is currently available. Prevention of these rodent-borne infections rests on control of the vector’s contact with humans. Most human cases of LCM in the United States have resulted from contact with pet hamsters or in rodent-breeding facilities. If hamsters must be kept as pets, scrupulous hand washing is recommended after contact.

Rabies
NIPAH VIRUS

In 1998, a newly emergent paramyxovirus was identified as the cause of an outbreak of encephalitis, with the first death occurring in the village of Nipah, Malaysia. The cases occurred in farmers and abattoir workers who had close contact with diseased pigs that had a respiratory illness. Nipah virus is closely related to another recently emerged paramyxovirus known as Hendra virus. Thus, the potential for new viral pathogens to emerge continues unabated.

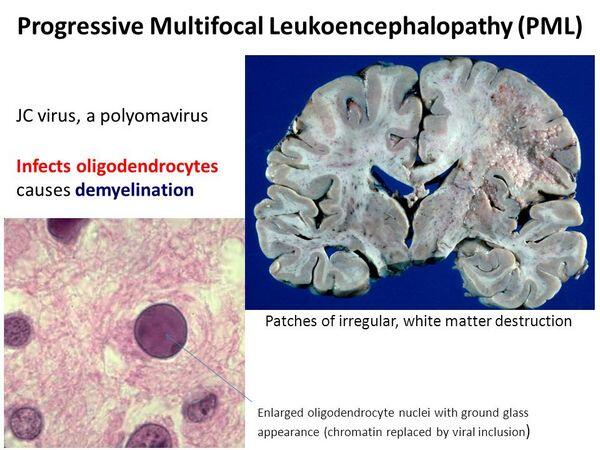
PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
Essentials of Diagnosis
- Progressive cerebral deterioration in an immunocompromised patient, leading to paralysis and death in < 1 year.
- Multiple lesions in white matter, as revealed by MRI.
- Virions visible on brain biopsy.
- Normal CSF findings (cell count, glucose, protein).
- JC virus (JCV) DNA detectable in CSF by PCR.
General Considerations
Epidemiology
Progressive multifocal leukoencephalopathy (PML) is a rare syndrome that occurs in immunocompromised patients, including those with AIDS, and is caused by a papovavirus known as JC virus (JCV). The virus was first recovered by coculturing of brain tissue from a patient with progressive multifocal leukoencephalopathy and Hodgkin’s disease. Polyomavirus infections are ubiquitous, and most humans are infected with JCV by the age of 15 years.
Microbiology
The polyomaviruses are small (44 nm in diameter), icosahedral, and lack an envelope. The double-stranded DNA is a circular, supercoiled molecule.
Pathogenesis
Respiratory transmission is the probable mode of spread. Immune suppression after organ transplantation or during pregnancy is capable of reactivating latent infections.
Clinical Findings
Signs and Symptoms
Clinical symptoms develop insidiously but progress relentlessly. Patients may have multiple neurologic symptoms unattributable to a single anatomic lesion. Impairment of speech, vision, coordination, mentation, or some combination of these occurs and is followed by paralysis of the arms and legs and finally death in approximately 1 year.
Laboratory Findings
CSF is normal and does not contain antibody to JCV but may be positive for JCV by PCR. Histologic examination of brain tissue from cases of progressive multifocal leukoencephalopathy reveals cytologic changes within the oligodendrocytes. These cells are adjacent to areas of demyelination. There is little if any inflammatory cell response. Electron microscopy can be used to visualize viral particles in brain tissue, and immunofluorescence can confirm the identity of viral antigen.
JCV grows best in primary human fetal glial cells, which are not readily available. Culture of JCV is therefore performed only in a few research laboratories.
Imaging
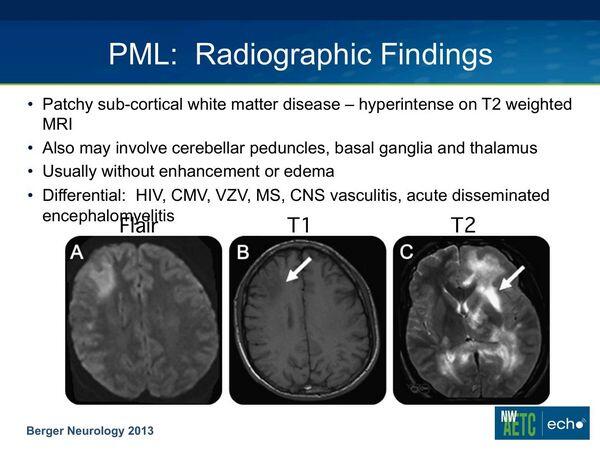
MRI is characteristic with multiple high signal-intense lesions predominantly in white matter.
Differential Diagnosis
Although the MRI is characteristic, similar clinical and radiologic findings can occur with VZV infections in AIDS patients.
Complications
There is progressive worsening of cerebral and neurologic function with death usually occurring 3-6 months from onset.
Treatment
No specific treatment is available, but some stabilization or improvement may occur if the immunosuppression can be reduced, eg, control of HIV infection.
Prognosis
Rarely there may be regression of symptoms especially if immunosuppression can be reduced. Otherwise the disease is fatal and may be especially rapid in AIDS patients.
Prevention & Control
The ubiquitous nature of polyomaviruses and the lack of understanding of their modes of transmission make preventing primary infection unlikely. Minimizing the duration and degree of immunosuppression can decrease reactivation of polyomavirus and the development of PML.
DISEASES CAUSED BY UNCONVENTIONAL AGENTS
Evidence has accumulated during the past 30 years that a variety of progressive neurologic diseases in both animals and humans are caused by viral or other filterable agents that share some of the properties of viruses. These illnesses have been termed slow viral diseases because of the protracted period between infection and the prolonged course of the illness, but a better term is persistent viral infection. Most persistent viral infections involve well-differentiated cells, such as lymphocytes and neuronal cells. These diseases are associated with unconventional viruses that are small, filterable infectious agents transmissible to certain experimental animals, but that do not appear to be associated with immune or inflammatory responses by the host and have not been cultivated in cell culture.
Viral persistence can result from integration of viral nucleic acid into the host genome, mutations that interfere with or severely limit viral replication or antigenicity, failure of host immune systems to recognize virus or infected cells, or some combination of these.
A group of progressive degenerative diseases of the central nervous system with similar pathology has been described. Two of the illnesses, Creutzfeldt-Jakob disease and kuru, occur in humans; two others, scrapie in sheep and goats and progressive encephalopathy in mink, occur in animals. Although the pathogenesis of these four illnesses is not well understood, there are various degrees of neuronal loss, spongiform neurologic changes, and astrocyte proliferation. The incubation periods are months to years, and the diseases have protracted and inevitably fatal courses.
The causes of these diseases are transmissible agents with unusual physical and chemical properties, but their nature is still obscure. They are small and filterable to diameters of 5 nm or less, multiply to high titers in the reticuloendothelial system and brain, produce characteristic infections, and can remain viable even in formalinized brain tissue for many years. They are resistant to ionizing radiation, boiling, and many common disinfectants. Recognizable virions have not been found in tissues, and the agents have not been grown in cell culture. Treatment of infectious material with proteases and nucleases does not decrease infectivity.
Brain extracts from scrapie-infected animals contain a glycoprotein called PrP that is not found in the brains of normal animals. PrP has been termed a prion (proteinaceous infectious particle), and purified proteinaceous extracts of brain tissue in very high dilutions have been shown to transmit disease to experimental animals. Repeated attempts to find associated nucleic acids have been generally unrewarding. PrP is encoded in a host gene, and specific prion mRNA has been found in both normal and infected tissue. Why the mRNA is translated in the disease and how prion production is apparently initiated by an external source of infectious PrP remain unanswered. During scrapie infection, prion protein may aggregate into birefringent rods and form filamentous structures termed scrapie-associated fibrils, which are found in membranes of scrapie-infected brain tissues.
Creutzfeldt-Jakob Disease
Table 1. Arthropod-borne viruses causing CNS disease.
Virus Family and Type
Vector
Hosts
Distribution
Disease
TOGAVIRUSES
Eastern equine encephalitis (EEE)
Aedis culiseta
Birds
North America (East and Gulf Coasts); South America
Mild systemic or severe encephalitis
Western equine encephalitis (WEE)
Culex culiseta
Birds
North and South America
Mild systemic encephalitis
Venezuelan equine encephalitis
Aedes culex
Small mammals; horses
North, South, and Central America
Mild systemic or severe encephalitis
FLAVIVIRUSES
St. Louis encephalitis
Culex spp.
Birds
North America
Encephalitis
Powassan
Ixodes ticks
Small mammals
North America
Encephalitis
Japanese encephalitis
Culex spp.
Pigs; birds
Asia
Encephalitis
West Nile
Culex spp.
Birds
Africa, Europe, U.S.
Fever, encephalitis, hepatitis
Russian spring- summer encephalitis
Ixodes and Derma- centor ticks
Birds
Russia
Encephalitis
BUNYAVIRUSES
California (La Crosse Virus)
Aedes spp.
Small mammals
Eastern one-half of North America
Mild systemic encephalitis
BOX 1. Other Viral Infections of the CNS
Children
Adults
More Frequent
WEE, EEE, CE
SLE
Less Frequent
SLE
WEE, EEE, VEE, West Nile Virus
Rare
Progressive multifocal leukoencephalopathy, rabies
Progressive multifocal leukoencephalopathy
Creutzfeldt-Jakob disease, rabies
BOX 2. Control of Arthropod-borne Viral Encephalitis
Prophylactic Measures
• Avoid mosquito and tick bites
• Vaccines for certain arboviruses
Isolation Precautions
• None
BOX 3. Treatment of Rabies
Children
Adults
First Choice
Treatment consists of immune enhancement: immune globulin and vaccine (see Box 5 for dosages)
Treatment consists of immune enhancement: immune globulin and vaccine (see Box 5 for dosages)
BOX 4. Control of Rabies
Prophylactic Measures
- Postexposure: immune globulin 20 IU per kg; infiltrate 1/2 into wound; vaccine 1.0 ml of human diploid cell vaccine (HDCV) or inactivated vaccine (RVA) on days 0, 3, 7, 14, and 28.
- Pre-exposure: vaccine
Isolation Precautions
- Not necessary for infected patient


