Essentials of Diagnosis
- Human immunodeficiency virus (HIV) RNA is detected and quantitated by polymerase chain reaction or branched DNA methods.
- Major diagnostic clues to HIV infection are a low CD4 lymphocyte count or an unexplained opportunistic infection.
- Human T-lymphotropic virus type 1 (HTVL-1) infection should be suspected in an adult patient with a T-cell malignancy or spastic paraparesis who is from Japan or the Caribbean basin.
General Considerations
Epidemiology
Two major groups of retroviruses are considered in this chapter: the oncoviruses (“onco-,” related to a tumor) and the lentiviruses (“lenti-,” slow). Oncoviruses have long been associated with a variety of cancers in animals, including leukemia, lymphoma, and sarcoma; however, until recent years, oncoviruses had not been found to infect humans. The first human retrovirus, human T-lymphotropic virus type 1 (HTLV-1), was discovered in the late 1970s. It was shown to cause adult T-cell leukemia, a rare malignancy found only in Japan, Africa, and the Caribbean (although serologic evidence shows that the virus also occurs in the United States and may be associated with some chronic neurologic conditions).
A related virus, HTLV-2, has been associated with some cases of human leukemia, including hairy cell leukemia, however its precise role in these diseases remains unclear. The most important disease resulting from human retrovirus infection—the acquired immunodeficiency syndrome (AIDS)—is caused by lentiviruses termed HIV-1 and HIV-2. This SITEfocuses on the virology of oncoviruses and lentiviruses and also describes the clinical presentations of the retroviruses other than HIV. See site for a discussion of the clinical presentation of HIV.
Microbiology
All retroviruses are remarkably similar in their basic composition. They are enveloped, single-stranded RNA viruses. They encode reverse transcriptase (an RNA-dependent DNA polymerase) that copies the genome into double-stranded DNA, which becomes integrated into the host cell genome. In addition to the structural proteins, the virion contains three virus-specific proteins that are essential for viral replication: reverse transcriptase, protease, and an integrase. The gag (group-specific antigen) gene encodes the structural proteins of the virus. The pol (polymerase) gene encodes the reverse transcriptase. The env (envelope) gene encodes the two membrane glycoproteins found in the viral envelope. Not surprisingly, the surface protein (gp120 in HIV-1) is responsible for the host range of the virus and its antigenicity.
Pathogenesis
Like most enveloped viruses, all retroviruses are highly susceptible to inactivation and are thus not transmissible through air, dust, or fomites under normal conditions; that is, they require intimate, direct contact with tissue or bodily fluids from the infecting source. Oncoviruses do not kill the cell they infect but instead usually continue to produce new virus indefinitely. This property, combined with the fact that oncoviruses can transduce growth-promoting genes called oncogenes into the recipient cell, accounts in part for the ability of oncoviruses to cause malignancies.
With lentivirus infections, the cell-virus relationship is quite different. Some lentiviruses can persist for years in a latent state without causing much cell killing, only to become highly cytolytic when the infected cells are subjected to certain stimuli. The prototype lentivirus is the visna virus, which causes a slowly degenerative neurologic disease in sheep. Other lentiviruses, for example, HIV-1, can persistently replicate at high levels resulting in cell death. Although HIV-1 can infect a variety of human cell types, its most drastic effects appear to result from destruction of the CD4+ subclass of T lymphocytes, which play a central role in the capacity of the host to mount effective immunologic responses to a wide range of infections.
VIROLOGY OF THE RETROVIRUSES
The genomes of transforming oncoviruses have a variety of structures, but one feature is common to nearly all of them. Some viral genes are replaced by genes derived from their hosts that render them oncogenic (see below).
A comparison of the genetic makeup of HIV-1 with that of other retroviruses reveals a larger number of genes and a much more complex organization. HIV-1 contains, in addition to the usual ensemble of genes, an array of other genes (tat, rev, nef, vif, vpr, and vpu). These additional genes apparently encode proteins that serve regulatory roles important in determining the long period of latency exhibited by the virus (see below). HTLV-1 encodes a similar array of regulatory proteins. To date, approximately nine subtypes of HIV-1 clads and five of HIV-2 have been discovered, based on their env and gag sequences.
Viral Entry
The virions adsorb to cellular membrane receptors and enter the cell probably by direct fusion with the plasma membrane. For HIV-1, the virion attachment protein is the surface glycoprotein gp120, and the cell receptor is the CD4 molecule that occurs primarily on the plasma membrane of CD4+ T lymphocytes, cells of the monocyte macrophage series, and some other target cells. The HIV-1 transmembrane protein gp41 is responsible for fusion of viral and cell membranes, a process that is important for entry of the virus into the host cell.
HIV-1 can also infect cells such as fibroblasts and certain brain cells that lack the CD4 surface molecule, apparently because the fusion-inducing activity of the transmembrane protein is sufficient in these cases to promote entry. An additional aspect of fusion activity is that infected cells expressing viral glycoproteins in their membranes readily fuse with uninfected CD4+ T lymphocytes to form large syncytia. This process appears to provide a means for cell-to-cell transmission of the virus that bypasses the usual extracellular phase and also damages the membrane of uninfected cells and affects their viability.
Viral RNA Replication
Among the RNA viruses, retroviral replication is unique. Soon after entry of the viral core into the cytoplasm of the infected cell, the RNA is copied into double-stranded DNA by reverse transcriptase, the virion-associated DNA polymerase. The process of reverse transcription results in a linear DNA molecule that enters the nucleus and integrates into the host cell chromosome. The viral integrase catalyzes the reaction required for the integration of the linear DNA into host DNA. Once the viral genetic information has been converted to DNA and integrated, it essentially becomes part of the cellular genome. The viral genes, called the provirus, replicate together with the host genome as long as the infected cell continues to divide.
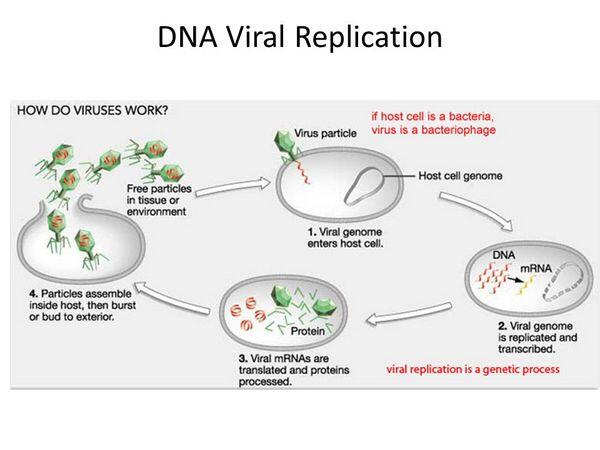
Special sequences contained within the RNA are duplicated during the reverse transcription process so that the integrated provirus contains identical long terminal repeats at its ends. The long terminal repeat sequences contain the appropriate promoter and enhancer and other signals required for transcription of the viral genes by the host RNA polymerase II.
Transcription produces both a full-length RNA genome and one or more spliced messenger RNAs (mRNAs). The predominant spliced mRNA is translated to produce the envelope glycoproteins, but, in HIV-1, a series of spliced mRNAs are produced that also encode a variety of viral regulatory proteins. With the exception of these regulatory proteins, all retroviral proteins are initially translated as polyproteins that are subsequently processed by proteolysis into the individual protein molecules. The enzyme responsible for most of these protein cleavages is the virus-specific protease that is encoded in either the gag gene or the pol gene of the virus. Of all the known retroviruses, HIV-1 possesses the most error-prone reverse transcriptase.
This property accounts for the many nucleotide differences observed between isolates (even from the same infected individual) and for the variability of the gp120 antigen. It may explain in part the failure of the immune system to control the infection and the increases in viral virulence that appear to occur during the course of the infection.
Unique Feature of the Lentiviruses
A unique feature of HIV-1 and other members of the lentivirus subfamily is the ability to produce a complex array of regulatory proteins. Although much remains to be determined about the regulatory circuits that modulate HIV-1 gene expression, several features of the process have been established.
Regulation appears to occur at three levels: mRNA production, transport of unspliced versus spliced mRNAs from the nucleus to the cytoplasm, and maturation of viral proteins during budding. Regulation may also occur at the level of mRNA translation. The HIV-1 regulatory proteins and their functions are listed in Table 40-1.
Two of the regulatory proteins, TAT and REV, play a positive role in promoting viral gene expression. The TAT protein increases the rate of viral mRNA synthesis, and REV promotes the transport of full-length mRNA to the cytoplasm before it can be spliced. The combined action of these two regulatory proteins is to promote the production of virus particles. The VIF protein promotes virus production at the level of maturation.
Superimposed on this complex regulatory network is the fact that the viral promoter contains elements that are sensitive to specific cellular transcription factors. This observation may help explain why virus production in CD4+ T lymphocytes is greatly increased when the cells are activated.
HIV Latency
Until recently, it was thought that the virus entered a latent stage in an infected host and became activated at a later date. Now it is understood that even during the asymptomatic period before clinical AIDS, the virus duplicates at a very high rate with a half-life of 2 days. Billions of HIV viruses are produced daily.
The long asymptomatic period after HIV infection (clinical latency) occurs despite active virus replication in the host. Several factors can terminate the long latent period of HIV-1. Mutations occur during viral replication that appear to increase cytopathic capacity and alter cell tropisms. In the same patient, the mutated forms of HIV-1 isolated from later stages of disease infect a broader range of cell types, grow more rapidly than those isolated in the asymptomatic period, and may induce syncytial formation (SI variants).
The virus replicates in macrophages, and these cells may serve as a reservoir for spread of the infection to other cell types by cell-to-cell fusion, which allows the virus to spread without being exposed to neutralizing antibodies. In addition to CD4+ T lymphocytes and macrophages, the most prominent cell types infected are glial cells and astrocytes in the brain and cells of the bowel mucosa. Infected macrophages may participate in the breakdown of the blood-brain barrier, allowing exposure of the central nervous system. Although central nervous system and intestinal disturbances are a prominent part of full-blown AIDS, it is not clear whether they are a direct result of infection of these cells or mediated by cytokines from infected macrophages and T lymphocytes.
The primary immune defect in AIDS results from the reduction in number and effectiveness of CD4+ helper-inducer T lymphocytes, both in absolute numbers and relative to CD8+ suppressor T lymphocytes. This reduction is caused by direct killing of CD4+ T lymphocytes by the virus but may also involve other mechanisms as well. These mechanisms include autoimmune processes that lead to the elimination of CD4+ T lymphocytes by opsonophagocytosis and antibody-dependent cell-mediated cytotoxicity directed at gp120 expressed on the CD4+ cell surface. There are also functional defects in CD4+ T lymphocytes affecting lymphokine production and leading to inhibition of some macrophage functions.
Effects on CD4+ T lymphocytes lead to a generalized failure of cell-mediated immune responses, but there is also an effect on antibody production caused by polyclonal activation of B cells, possibly associated with other viral infections of these cells. This overwhelms the capacity of infected individuals to respond to specific antigens. The result of these processes is a disturbance of immune balance that can give rise to malignancies as well as to a range of opportunistic viral, fungal, and bacterial infections.
Transformation by Retroviruses
Oncogenic retroviruses appear to transform cells to an oncogenic state by three distinct mechanisms. First, the transforming viruses have acquired a cellular gene (called an “oncogene”) that, when expressed in the infected cell, results in the loss of normal growth control. On infection, the oncogene is expressed, resulting in the rapid onset of malignant disease. Persistent transformation by oncogene transduction is possible only for those retroviruses that are not cytocidal. More than 25 different oncogenes have been identified in a variety of animal retroviruses, but no human retroviruses are known that transform by this mechanism.
The second mechanism is called “insertional mutagenesis.” Integration of a retrovirus near particular cellular genes can cause inappropriate expression of the gene, resulting in uncontrolled cell growth. These cellular genes are called “proto-oncogenes,” and insertional activation by the virus is apparently caused by the close proximity of the integrated viral promoter or enhancer to the gene. Cancers that are caused by this mechanism have very long latent periods because integration is random and only rarely occurs near a cellular proto-oncogene. No human cancers are known to be caused by this mechanism.
The causative agent of adult T-cell leukemia, HTLV-1, exemplifies the third mechanism. In this case, the integrated provirus in the leukemic cells from any one patient is found at a unique location on a particular chromosome. Thus the tumors are probably monoclonal. However, the cancer is not the result of insertional mutagenesis because the chromosomal location of the provirus is never the same in any two patients. Instead, transformation results from the continual expression of the viral tax gene (the HTLV-1 homolog of the HIV-1 tat gene). Apparently, the TAX protein not only can activate viral transcription in the same manner as TAT but can also activate the expression of one or more cellular genes (possibly proto-oncogenes) resulting in malignant transformation. These leukemia viruses cause cancer after a long latency period.
ONCOVIRUSES (HTLV-1)
Clinical Findings
HTLV-1 infection is usually asymptomatic but can progress to adult T-cell leukemia in ~1 in 20 persons (Box 40-1). Adult T-cell leukemia caused by HTLV-1 is a neoplasia of CD4 helper T cells that can be acute or chronic. In addition to an elevated and abnormal lymphocyte count, lymphadenopathy, splenomegaly, and hepatomegaly also occur. The skin lesions vary from localized maculas, papule nodules, and plaques to generalized erythroderma. Adult T-cell leukemia is usually fatal within 1 year of diagnosis, regardless of treatment. A second syndrome associated with HTLV-1 infection is tropical spastic paraparesis. This is a slowly progressing neurologic disorder that usually begins as bilateral weakness of the lower extremities with hyper-reflexia. Numbness, back pain, and symptoms of bladder irritation also develop.
HTLV-1 infection is detected immunologically by the presence of HTLV-1 antibody in blood. A diagnosis of adult T-cell leukemia is supported by a high number of abnormal lymphocytes in blood and is confirmed by identification of HTLV-1 DNA in these cells.
Treatment & Control
Adult T-cell leukemia may respond briefly to chemotherapy, but no treatment has been proved effective against HTLV-1 infection. Zidovudine (azidothymidine [AZT]) and other inhibitors of reverse transcriptase are active against HTLV-1 in tissue culture, but controlled studies are needed.
Table 1. Human immunodeficiency virus (HIV) and human T-lymphotropic virus (HTLV) regulatory proteins.
Gene
Protein
Function
HIV tat
TAT
Increases rate of viral transcription.
HTLV tax
TAX
Same as for TAT.
HIV rev
REV
Increases transport of unspliced mRNAs.
HTLV rex
REX
Same as for REV.
HIV nef
NEF
Negative regulator of transcription.
HIV vif
VIF
Facilitates virus maturation during budding.
BOX 1. Oncovirus (HTLV) Infections
Children
Adults
More Common
- Clinical syndromes not identified in children
- Adult T-cell leukemia
Less Common
- Tropical spastic paraparesis
BOX 2. Treatment of Oncovirus (HTLV) Infection
Adults
First Choice
- Chemotherapy for malignancy
Second Choice
- Antiretroviral agents (investigational only)
BOX 3. Control of Oncovirus (HTLV) Infection
Prophylactic Measures
- As with HIV, prevention is best accomplished by eliminating exposure to bodily fluids, especially blood and semen.
Isolation Precautions
- Eliminate exposure to bodily fluids, especially blood.