Essentials of Diagnosis
- Key symptoms include initially profuse and watery diarrhea progressing to foul-smelling and often greasy stools that float.
- It is the most common pathogen in waterborne diarrheal illness.
- Patients at highest risk include infants, young children, travelers, and immunocompromised patients.
- In North America, the Rocky Mountains and mountainous regions of the northwest, northeast, and British Columbia are notorious Giardia reservoirs.
- Giardiasis is diagnosed either by identification of cysts or trophozoites on wet mounts of fresh stool or duodenal specimens or by antigen detection using enzyme-linked immunosorbent assay or immunofluorescence techniques.
General Considerations
Giardia, a genus of primitive eukaryotes, is a flagellated enteric protozoan of the class Zoomastigophorea. Giardia lamblia, also known as Giardia intestinalis or Giardia duodenalis, is the species known to infect humans. Its name comes from Vilem Lambl, who first reported the organism in 1859. However, the first description of G lamblia came from Anton von Leeuwenhoek in 1681, while examining his own stool during an episode of diarrhea.
Epidemiology
G lamblia is a global enteric pathogen. It is the most prevalent enteric parasite in the United States and Canada, and populations at highest risk include infants, young children, travelers, and immunocompromised patients. Between 1965 and 1984, the Centers for Disease Control and Prevention documented 90 outbreaks, making G lamblia the most common pathogen in waterborne diarrheal illness. Giardiasis plays a role in malnutrition and growth retardation in the developing world.
In some areas of the developing world, the overall prevalence of Giardia infection is as high as 20-30%, whereas prevalence in industrialized nations is 2-5%. Age-specific prevalence increases from infancy through childhood before falling in adolescence, and children < 10 years old in developing nations have a 15-20% prevalence of G lamblia infection. The highest giardiasis prevalence is seen in the subtropics and tropics. G lamblia accounts for < 5% of traveler’s diarrhea, with increased risk after travel to southeast and south Asia, tropical Africa, Mexico, South America, and areas of the former Soviet Union, particularly St. Petersburg. In North America, the Rocky Mountains and the mountainous regions of the Northwest, Northeast, and British Columbia are notorious G lamblia reservoirs.
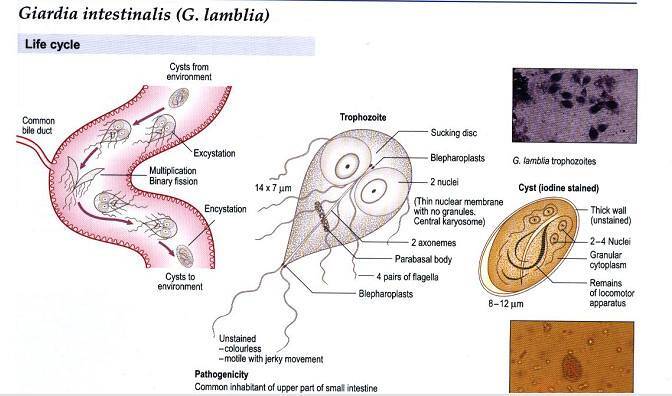
G lamblia is ingested orally, and transmission has been associated with contaminated water, person-to-person spread, and, less often, food-borne transmission. Most outbreaks are related either to untreated water or to inadequately purified water. The G lamblia cyst is particularly well suited to survive in cold water and is relatively resistant to chlorine.
Person-to-person transmission is related to poor fecal-oral hygiene. Children in day care facilities have an infection prevalence of = 50%, and sexually active male homosexuals, regardless of HIV status, have a prevalence of 20%. Increased numbers of infections are also found in individuals in custodial situations. Food-borne cases related to infected food handlers have been increasingly reported.
Studies have confirmed the presence of a vast animal reservoir. The first associations involved the beaver as a source of water contamination. Subsequently, DNA similarities have been found in Giardia isolates from humans and both domestic and wild animals, including beavers, cattle, cats, coyotes, dogs, gerbils, and sheep.
Microbiology
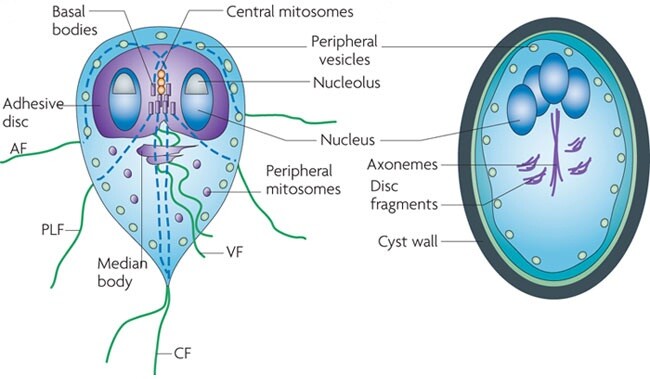
The G lamblia life cycle has two stages, the trophozoite and the cyst. The pear-shaped trophozoite lives freely in the small bowel lumen and is 9-21 um long × 5-15 um wide. It has a convex dorsal surface, a flat ventral surface with a sucking disk, and four pairs of posterior flagella. Absorption of nutrients occurs through the dorsal surface. The sucking disk is composed of an array of microtubules containing tubulin, microribbons containing the protein giardin, and other contractile proteins. Within the trophozoite is a posteriorly placed median body and two anterior nuclei each with a prominent karyosome, giving G lamblia its characteristic facelike appearance. The trophozoite divides by binary fission, doubling in 9-12 h in culture. An aerotolerant anaerobe lacking mitochondria, the trophozoite scavenges phospholipids, fatty acids, cholesterol, and pyrimidine. It metabolizes glucose to ethanol, acetate, and carbon dioxide. In vitro growth is optimized by conditions comparable with those found in the small intestine, including the presence of biliary lipids, intestinal mucus, epithelial cells, and low oxygen tension.
Encystation occurs in the small bowel, possibly because of high concentrations of bile salts and elevated pH. The highly resistant cyst is passed out of the host into the environment where trophozoite division occurs within the cyst. The mature G lamblia cyst is an oval structure (8-12 um long × 7-10 um wide) with four nuclei and an acid phosphatase-positive periphery encased in a thin wall that is composed primarily of N-acetylgalactosamine. Hundreds to thousands of cysts may be excreted per gram of stool. After ingestion and exposure to gastric acid and pancreatic enzymes, excystation releases two trophozoites to resume the cycle.
Pathogenesis
G lamblia infection requires the oral ingestion of as few as 10 cysts. Excystation, promoted by gastric acid, releases trophozoites, which then multiply and colonize the upper small bowel. Trophozoites attach to the brush border enterocytes by two proposed mechanisms. First, the ventral disk may be involved in attachment by either contractile proteins or flagellum-mediated hydrodynamic forces. Second, a receptor-ligand interaction mediated by lectin has been suggested. The attachment process enables the trophozoite to avoid peristalsis.
The exact mechanism of injury causing disease is uncertain, but several observations have been made. First, the brush border is disrupted by microvilli injury and villous atrophy, which cause a disaccharidase deficiency. It has been postulated that this injury may be caused by a proteinase or mannose-binding lectin. Second, increased epithelial turnover in the crypts has led to altered absorption, which may be caused by immature enterocytes. T lymphocytes may contribute to this crypt hyperplasia, which is also observed in graft vs host disease. Third, decreased bile salt concentrations with consequent diminished pancreatic lipase activity and impaired solubilization of fat has been reported in giardiasis patients. The trophozoite, although unable to deconjugate bile salts, does have an uptake mechanism for bile salts that, in low concentrations, stimulate growth. Low-bile-salt concentrations in giardiasis patients may also result from deconjugation by simultaneous colonization with Enterobacteriaceae or yeasts. This increased colonization of anaerobic and aerobic bacteria in giardiasis has not been uniformly reported, however, with all confirmatory studies coming only from India. Fourth, G lamblia infection inhibits trypsin. Thus, disaccharidase deficiency, immature enterocytes, and both lipase and trypsin inhibition suggest that the diarrhea in giardiasis is primarily malabsorptive. Evidence supports neither mucosal invasion nor the presence of an enterotoxin in the pathogenesis of giardiasis.
The immune response to G lamblia infection is initiated by antigen uptake into macrophages in Peyer’s patches. This action generates both an antibody and a cellular response. Although serum immunoglobulins M and G are lethal to G lamblia by the classical complement pathway, secretory immunoglobulin A (IgA) appears to be more important in clearing and preventing infection. Intraluminal IgA can prevent adherence, and chronic giardiasis is associated with the failure to make IgA. G lamblia has been found to make an IgA protease that is protective to trophozoites.
A cellular immune response is also generated and shown in mice to be necessary for both cytotoxicity and coordination of IgA secretion. As already mentioned, the T-cell response may also contribute to the pathogenesis of G lamblia because the mononuclear cell submucosal infiltrate is associated with flattened villi and crypt hypertrophy.
Protective immunity does not develop after a single infection, possibly because of genomic plasticity and significant antigenic diversity described in G lamblia isolates. However, increased prevalence in the young and decreased symptoms in long-term residents of endemic areas suggest at least partial immune protection. Infection in infants < 6 months old is rare, and human milk is protective because of the presence of antibodies and cytotoxicity from free fatty acids generated from milk triglycerides.
Although occurring in immunocompetent hosts, a predisposition to chronic giardiasis is reported in patients with X chromosome-linked agammaglobulinemia, lymphoid nodular hyperplasia, and common variable immunodeficiency with variable levels of hypogammaglobulinemia. Patients with earlier gastric surgery and decreased gastric acidity also have an increased susceptibility to infection. Of interest, patients with AIDS have no more severe illness than patients without AIDS, in contrast to the disparity seen in intracellular protozoal infections such as Cryptosporidium parvum.
Table 1. Laboratory diagnosis of giardiasis.1
- The trophozoite is 10 µm × 15 µm with a convex dorsal surface, flat ventral surface, and 2 facelike nuclei
- The cyst is 10 µm × 10 µm, thin-walled, and with an eccentric nucleus
- Motile trophozoites are seen on microscopy of fresh stool specimens or on specimens obtained by duodenal aspirate, biopsy, or the string test
- Cysts and trophozoites are observed on wet mounts of fresh stools with or without prior formalin concentration. Cysts can also be found in specimens preserved in formalin or PVA, using trichrome or iron hematoxylin staining
- Antigen detection by ELISA is highly sensitive and specific and comparable in cost to standard stool O & P. It is best used when the sole diagnosis or exclusion of giardiasis is needed
- Antigen detection by IFA has the same sensitivity and specificity as standard O & P, but is advantageous in small labs with less-trained technicians
- DNA probes and PCR techniques are available but not yet useful clinically
1O & P, Ova and parasite examination; PVA, polyvinyl alcohol; IFA, immunofluorescent antibody assay
BOX 1. Giardiasis
Acute
Chronic
More Common
- Diarrhea
- Malaise
- Flatulence
- Abdominal cramps
- Diarrhea
- Alternating diarrhea with constipation
- Abdominal pain worsening by eating
- Malaise
Less Common
- Nausea
- Weight loss
- Vomiting
- Urticaria
- Malabsorption
- Macrocytic anemia
- Lactose intolerance
- Weight loss
- Headache
BOX 2. Treatment of Giardiasis
Adults
First Choice
- Metronidazole, 250 mg three times daily × 5-7 d
OR
- Tinidazole1 2 g × 1
OR
- Quinacrine (Mepacrine)1 100 mg three times daily × 5 d
Second Choice
- Furazolidone, 100 mg four times daily × 7-10 d
OR
- Paromomycin, 25-30 mg/kg/d divided three times daily × 5-10 d
Children
- Metronidazole, 5 mg/kg three times daily × 7 d
OR
- Furazolidone liquid, 6 mg/kg divided 4×/day for 10 d
Pregnancy
- Delay until after delivery if possible
- Metronidazole may be used after first trimester — see above
- Paromomycin, see above for dosing
Not available in the United States.
BOX 3. Prevention & Control of Giardiasis
Prophylactic Measures
- Proper treatment of public water supplies including chlorination, flocculation, sedimentation, and filtration
- In the wilderness, bring water to a rolling boil
- Halogenation with chlorine or iodine tablets may be effective with warm water
- Filters with < 1-µm pores can be used as well
Isolation Precautions
- No specific need for isolation
- Good hygiene practices