FUSARIUM INFECTION
Essentials of Diagnosis
- Worldwide geographic distribution.
- Mold, septate hyphae 3-8 um in diameter.
- A rare infection in severely immunocompromised patients.
- Blood cultures often but not always positive.
- No serologic tests available.
- Cutaneous involvement is common feature.
General Considerations
Epidemiology
Fusarium spp. is an emerging fungal pathogen. Although long recognized as a cause of local infection involving nails, traumatized skin, or the cornea (eg, in contact lens wearers), deep or disseminated infection was not described until the mid 1970s. Despite its worldwide distribution and its frequent recovery from soil and vegetative material, infection is quite rare. Only ~ 100 cases involving invasive disease in immunosuppressed patients have been described in the medical literature.
Most cases of serious disease occur in patients with hematologic malignancy and chemotherapy-induced neutropenia. Profound neutropenia and prolonged neutropenia increase the risk for infection. The respiratory tract is the most common portal of entry leading to infection, but other sites of acquisition include a disrupted skin barrier, the digestive tract, and a central venous catheter. Fusarium onychomycosis also appears to be a potential source of disseminated infection in at-risk patients.
Microbiology
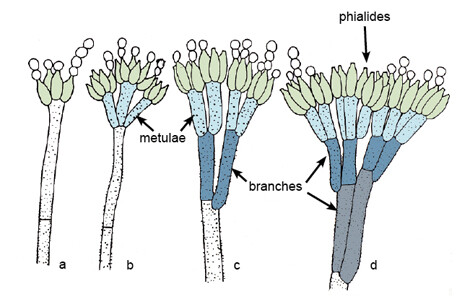
Fusarium spp. are filamentous fungi that are soil saprophytes and plant pathogens. The hyphae range in size from 3 to 8 um and are generally septate. Growth from clinical specimens usually occurs after 4-5 days of incubation in the laboratory. Dichotomous branching is common, and branches usually occur at an angle of 45°. Consequently, Fusarium spp. are indistinguishable from Aspergillus spp. (see site) when seen in tissue. Although there are many different species, human disease appears to be limited to F oxysporum, F solani, F moniliformie, and F proliferatum. Like other filamentous fungi, the different Fusarium spp. may be identified by the distinguishing characteristics of their fruiting bodies and microconidia.
Pathogenesis
The clinical pathogenic pattern of infection caused by Fusarium spp. is very similar to that of Aspergillus infection. Although much rarer than invasive aspergillosis, the same pathogenic mechanisms that allow A conidia to invade tissue contribute to the development of Fusarium infection. Alteration in macrophage function by steroid therapy and neutropenia allow the Fusarium microconidia to replicate unimpaired in host tissue. Angioinvasion leads to early dissemination particularly to skin structures. The reason for the marked propensity to disseminate to skin is unknown. Colonization with Fusarium spp. without infection is common, particularly among burn patients.
Clinical Findings
Signs and Symptoms
Invasive fusariosis usually develops with the abrupt onset of high spiking fevers (Box 1). Although myalgias are a common feature, infection of muscle is uncommon but has been reported. Focal symptoms may depend on the site of infection. Shortness of breath, cough, and hemoptysis are present in patients with pulmonary involvement. Central venous catheter infection may be associated with tunnel tract tenderness or exudation.
Physical findings are also dependent on the extent of invasive disease and sites of dissemination. Almost 80% of patients will have cutaneous involvement. Typically, this will begin as a erythematous indurated maculopapular lesion, which will enlarge and eventually ulcerate, leaving a nodular lesion with a necrotic, often black center. Multiple lesions are commonly seen and may resemble ecthyma gangrenosum. Localization to the extremities is most common. Primary cutaneous fusariosis may present with a single necrotic skin lesion. Multiple skin lesions suggest disseminated disease. A rhinocerebral form of infection has been described that is indistinguishable from rhinocerebral mucormycosis or aspergillosis.
Laboratory Findings
Neutropenia is usually present at the onset of the signs and symptoms of infection, but there are no other commonly associated hematologic or chemistry abnormalities. Blood cultures are positive in 60% of patients with invasive fusariosis, which is in marked distinction to invasive aspergillosis, where blood cultures are negative. The reason for this difference is unknown. There are no serological tests available for diagnosing Fusarium infection.
Imaging
The chest roentgenogram in a patient with pulmonary involvement may show nonspecific infiltrates, nodular lesions, or cavitary lesions. With rhinocerebral infection, opacification of one or all the sinus cavities and destruction of bony structures can best be imaged with computed tomography. A bone scan may be helpful to determine if there is bone involvement underlying extensive cutaneous involvement.
Differential Diagnosis
In addition to invasive fusariosis, the differential diagnosis of persistent neutropenic fever includes invasive aspergillosis and other infection with opportunistic filamentous fungi. Although Candida, Aspergillus, and Rhizopus spp. are all capable of causing similar skin lesions in disseminated invasive disease, Fusarium spp. are the most likely of these organisms to do this. Histologically and clinically, fusariosis resembles invasive aspergillosis and infection due to Pseudoallescheria boydii (see site).
Complications
Widespread or metastatic cutaneous involvement can develop from a single primary focus of infection. Disseminated disease may involve the heart, liver, kidneys, spleen, brain, and other vital organs.
Diagnosis
The diagnosis is confirmed by the recovery of Fusarium isolates from blood or biopsy specimens. Skin lesions developing in the setting of new fever in a neutropenic patient should be biopsied for histopathology and culture. The recovery of Fusarium spp. from sputum can suggest the diagnosis in the appropriate host with a syndrome compatible with invasive fusariosis; however, colonization of the respiratory tract can occur with this organism.
Treatment
Amphotericin B is the treatment of choice (Box 2). Response to therapy is usually poor, and in vitro susceptibility testing suggests that more than half of the strains tested are resistant to amphotericin B. It is unknown if the lipid preparations of amphotericin B offer any therapeutic advantage, but patients have been successfully treated with lipid preparations of amphotericin B. The use of granulocyte colony stimulating factors and granulocyte transfusions has been reported to be effective in some patients. Fusarium spp. are uniformly resistant to 5-flucytosine. The role of treatment with azoles is unknown. The most important factor contributing to successful treatment is recovery of neutropenia.
Prognosis
Invasive fusariosis is a lethal disease. The overall mortality rate according to the medical literature is > 70%.
Prevention & Control
Because the skin and nails are potential portals of entry, before chemotherapy-induced neutropenia, patients with significant onychomycosis should be evaluated by a dermatologist for Fusarium infection (Box 3). Futhermore, high-risk neutropenic patients with skin trauma and contamination should be carefully examined for potential inoculation with Fusarium spp. This may need to include biopsy and culture.
BOX 1. Fusariosis in Adults and Childrenx
More Common
- High fever, acute onset
- Myalgias
- Shortness of breath, hemoptysis, cough
- Cutaneous involvement
- Positive blood culture
Less Common
- Rhinocerebral infection
- Central nervous system involvement
- Intra-abdominal organ involvement
BOX 2. Treatment of Fusariosis in Adults and Children
First Choice
Amphotericin B, 1-1.5 mg/kg/d until neutropenia resolved and full response
Second Choice
Lipid preparation of amphotericin B (dose dependent on specific preparation)
Comment
- Treatment with granulocyte colony stimulating factor and granulocyte transfusion should be considered
- Many strains are amphotericin B resistant
- Recovery of neutropenia is critical
BOX 3. Control of Fusariosis
Prophylactic Measures
- Evaluate patients with significant onychomycosis for fusarium infection prior to chemotherapy
- High-risk patients with skin trauma and contamination should be examined for inoculation with Fusarium spp.
Isolation Precautions
None
BOX 4. Penicillium marneffei Infection in Adults and Children
More Common
- Chronic illness
- Fever
- Weight loss
- Cutaneous involvement
- Generalized lymphadenopathy
- Anemia
- Positive blood culture
Less Common
- Diarrhea
- Pericarditis
- Bone and joint involvement
- Central nervous system involvement
BOX 5. Treatment of Penicillium marneffei Infection in Adults and Children
First Choice
- Amphotericin B, 0.5-1.0 mg/kg/d for 2 wk, followed by 6 wk of itraconazole, 200 mg daily in adults or 3-5 mg/kg daily in children
Second Choice
- Itraconazole, 200 mg daily in adults or 3-5 mg/kg daily in children for 2 months
Comment
- 5-flucytosine, 100-150 mg/kg in 4 divided doses daily, may be given in addition to amphotericin
- More severe cases may need a more prolonged course of amphotericin ± 5-flucytosine
- Chronic maintenance therapy is indicated in patients with AIDS
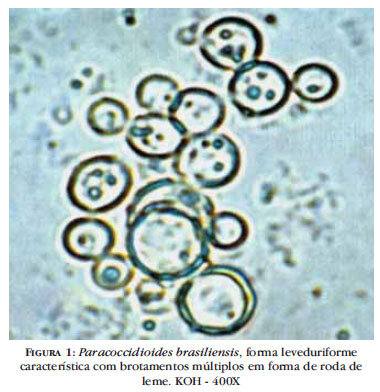
BOX 6. Paracoccidioidomycosis in Adults and Children
More Common
- Chronic illness
- Fever
- Mucocutaneous involvement (mouth, nose, larynx)
- Cutaneous involvement
- Lymphadenopathy
- Pulmonary involvement
Less Common
- Hepatosplenic disease
- CNS involvement
- Bone and joint involvement
- Arteritis
BOX 7. Treatment of Paracoccidioidomycosis in Adults and Children
First Choice
- Ketoconazole, 400 mg/d for adults or 5-10 mg/kg daily for children, for 6-18 months or itraconazole, 100 mg/d for adults or 3-5 mg/kg daily in children, for 6 months
Second Choice
- Amphotericin B, 0.5-1.0 mg/kg/d, total cumulative dose of 1-3 g in adults or 25-30 mg/kg in children
- Sulfadiazine, 2-6 g/d in adults or 120-150 mg/kg in children, for 4 wk followed by 500 mg/d in adults or 25-50 mg/kg/d in children, for 3-5 y
Comment
- If amphotericin B is used as initial therapy, itraconazole or sulfadiazine should be given as maintenance therapy, as above
- Trimethoprim-sulfamethoxazole may be substituted for sulfadiazine and should be given as lifelong maintenance therapy in patients with AIDS
- Fluconazole may be as effective as itraconazole
BOX 8. Control of Paracoccidioidomycosis
Prophylactic Measures
Trimethoprim-sulfamethoxazole
Isolation Precautions
None
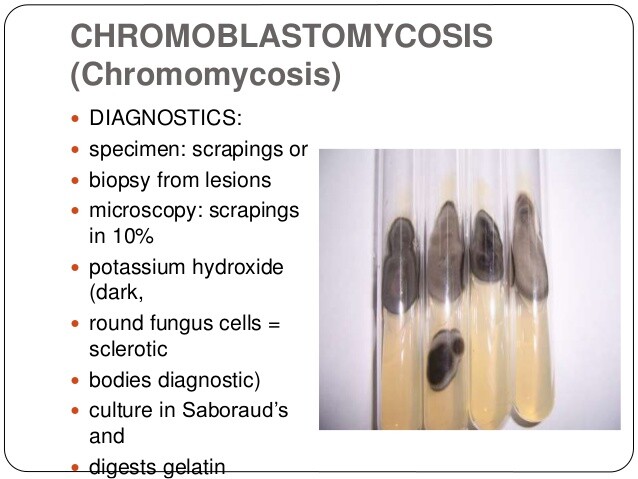
BOX 9. Chromomycosis in Adults and Children
More Common
- Chronic infection
- Foot involvement
- Cauliflowerlike appearance
Less Common
- Hand, arm, leg involvement
- Marked lymphedema
- Dissemination (very rare)
BOX 10. Treatment of Chromomycosis in Adults and Children
First Choice
- Wide surgical excision or cryotherapy with liquid nitrogen
Second Choice
- Itraconazole, 100 mg/d x 18 mo; for children, 5-6 mg/kg/d
- 5-Flucytosine, 100-150 mg in 4 divided doses/d × = 18 mo; for children, 50-150 mg/kg/d
Comment
- Terbinafine may be an option; more data needed
- Results with fluconazole have been disappointing but 400 mg/d is an option; for children, 3-6 mg/kg/d
- Failure to respond to antifungal therapy is often seen
BOX 11. Control of Chromomycosis
Prophylactic Measures
- Foot infection can be prevented by wearing shoes.
- At-risk individuals (eg, agricultural workers) should minimize trauma to hands and extremities.
Isolation Precautions
None