Essentials of Diagnosis
- Nosocomial acquisition.
- Predisposing factors include immunosuppression (neutropenia, cystic fibrosis [CF], AIDS, corticosteroid use, diabetes mellitus); presence of a foreign body, prosthesis, or instrumentation; prolonged hospitalization and antibiotic use; intravenous drug use.
- Most common infections include pneumonia, bacteremia, urinary tract infection, otitis media, skin and skin structure infections, including ecthyma gangrenosa.
- Gram stain shows gram-negative bacilli; recovery of microorganism from culture of blood or other tissue.
General Considerations
Epidemiology
The genus Pseudomonas consists of a number of human pathogens, the most important of which is Pseudomonas aeruginosa. P aeruginosa is an opportunistic pathogen found widely in soil, water, and organic material, reflecting its limited nutritional requirements. A moist environment is favored. Human colonization in the community is rare, and, when it occurs, the skin, gut, and upper or lower airway are colonized. Carriage of P aeruginosa in the community is associated with predisposing medical conditions, which allow the organism to bridge normal host defenses such as loss of the normal mechanical barrier provided by skin encountered in burn patients or abnormalities in pulmonary physiology encountered in patients with bronchiectasis or CF. In addition, prolonged antibiotic use by eliminating the normal host flora predisposes to P aeruginosa colonization.
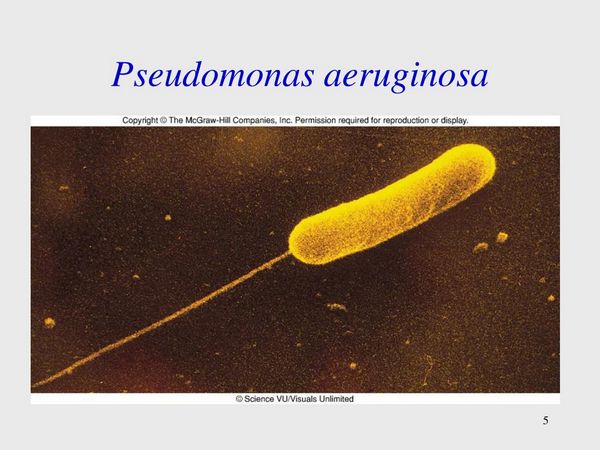
Nosocomial colonization is much more frequent and most often occurs in patients with predisposing conditions leading to impaired immunity, especially neutropenia, the presence of instrumentation disrupting host defenses, the prolonged use of extended spectrum antibiotics, and the existence of hospital reservoirs of infection. Hospital epidemics have been investigated by a variety of epidemiologic tools including serotyping, antibiogram patterns, and phage typing, but increasingly DNA fingerprinting is used. Potential sources of infection in the hospital environment include infected respiratory equipment, endoscopes, infusion solutions, intravascular catheters, whirlpools, sinks, drains, and indoor plants.
Microbiology
On the basis of ribosomal RNA and DNA sequence homology, the Pseudomonas genus is divided into five groups. Group I contains P aeruginosa in addition to P fluorescens and P alcaligenes; Group II contains P mallei, P pseudomallei, and Burkholderia cepacia; Group III contains the Comamonas species; Group IV contains P diminuta; and Group V contains Stenotrophomonas maltophilia (formerly Xanthomonas maltophilia).
Pseudomonas spp. are gram-negative, straight or slightly curved rods that are motile by means of flagella. They are nonsporing and facultative aerobes. Table 1 outlines the essential microbiological features of P aeruginosa by which it can be distinguished from other non-Pseudomonas gram-negative bacteria. Differentiation from other Pseudomonas spp. is more difficult and uses differences in sugar oxidation, growth characteristics at 42 °C, and flagella stains. The production of the pigment pyocyanin by approximately half of P aeruginosa strains is specific, and a sweet grapelike odor is often said to be characteristic of P aeruginosa.
Pathogenesis
The pathogenicity of P aeruginosa is a function of host and microbial factors. P aeruginosa is an opportunistic pathogen. Host defects may result from alterations in the normal physical barriers to infection. These may be breached by physical alterations such as occur to the skin in burn patients or by prosthetic materials such as catheters that bypass these mechanical barriers. The presence of an effective immune response to P aeruginosa requires functional neutrophils in adequate number, the presence of specific immunoglobulin G (IgG) antibodies, and complement activation by both classical and alternative pathways.
The production of high titers of antibody to exoenzyme A early on in P aeruginosa septicemia results in an improved outcome as compared with a less favorable outcome in those patients with low titers of antibody to exoenzyme A. Immunological defects contribute to infection and may consist of neutropenia, hypogammaglobulinemia, complement defects, or more subtly, age, prolonged hospitalization, or diabetes. Bronchiectasis and CF are lung diseases associated with pathological changes that predispose to colonization and often infection with P aeruginosa.
Bacterial factors also contribute. Colonization often precedes infection. Colonization is aided by the bacterial pili and production of mucoid exopolysaccharide. Pili or fimbriae are of particular importance in pulmonary colonization. The alginate capsule is a feature of mucoid strains and has an important role in CF pathogenesis (see below). It protects the organism from antibodies, complement, and phagocytosis. In addition, mucoid strains are associated with decreased susceptibility to antibiotics, especially aminoglycosides. Enzymes that contribute to invasiveness are produced, including alkaline protease, hemolysins, and elastase.
Alkaline protease induces necrosis in tissues, possesses strong anticoagulant activity, and inactivates a variety of cytokines including tumor necrosis factor (TNF). Elastase has a variety of pathogenic effects including the degradation of IgG and IgA, complement cleavage, inactivation of TNF, and interferon gamma, and is linked to the pathogenesis of the characteristic skin lesions of Pseudomonas septicemia referred to as ecthyma gangrenosum.
Hemolysins aid tissue invasion by degrading lipids and lecithin. One hemolysin, phospholipase c, degrades phosphatidylcholine, a component of lung surfactant, resulting in atelectasis. Cytotoxin, formerly called leukocidin, inhibits neutrophil function and is linked to the pathogenesis of Pseudomonas-induced acute lung injury in adult respiratory distress syndrome. Pyocyanin alters the function of ciliated respiratory epithelium and enhances tissue damage by means of the generation of toxic free radicals.
As with other gram-negative bacteria, lipopolysaccharide plays a key role in the manifestations of septic shock. It stimulates the production of TNF and other cytokines, prostaglandins, leukotrienes, ß-endorphins, kinins, complement activation, and the activation of the coagulation and fibrinolytic cascades. Exotoxin A acts by a method similar to diphtheria toxin to inhibit protein synthesis.
This toxin plays a significant role in the necrosis observed in animal models of Pseudomonas corneal or lung injury. Mutant strains of bacteria that do not produce exotoxin A produce less severe injury than strains expressing exotoxin. Exotoxin A may also be immunosuppressive to both T and B lymphocytes. Exoenzyme S ribosylates proteins of the ras gene superfamily and alters local host defense mechanisms.
Clinical Syndromes
Box 1 summarizes the principal clinical symptoms associated with P aeruginosa infection.
Infections in Patients With Cystic Fibrosis
BACTEREMIA
P aeruginosa is a common cause of nosocomial bacteremia, which may be either primary (no identifiable source) or secondary (recognizable extravascular source). Community-acquired P aeruginosa bacteremia is very rare. Host factors contributing to nosocomially acquired P aeruginosa bacteremia include neutropenia caused by hematological malignancy or chemotherapy, hypogammaglobulinemia, AIDS, organ transplantation, insulin-dependent diabetes mellitus, burns, premature births or advanced age, instrumentation or catheterization, high-dose corticosteroid use, and prolonged antibiotic therapy. P aeruginosa bacteremia is associated with higher mortality than bacteremia caused by other gram-negative microorganisms, although this observation may reflect the host’s underlying immunosuppression.
A poor prognosis is associated with an absolute neutrophil count of < 100 cells/mL3, septic shock, renal failure, or a serious underlying illness. Primary bacteremia or bacteremia-complicating pneumonia or skin infections are also associated with a poor prognosis. Clinical signs of P aeruginosa bacteremia are those of gram-negative sepsis and include fever, tachycardia, respiratory distress, hypotension, obtundation, and renal failure. P aeruginosa bacteremia has a particular propensity to cause jaundice. Disseminated intravascular coagulation is less frequently encountered with P aeruginosa bacteremia than other gram-negative bacteremias.
The classic skin lesion of P aeruginosa bacteremia is ecthyma gangrenosum, which is characterized by the presence of a small vesicle with a rim of surrounding erythema that undergoes necrosis and ulcerates with localized gangrene and black discoloration. Histopathology reveals the invasion of small arteries and veins by bacteria and is characterized by the absence of a significant inflammatory infiltrate. Lesions occur most frequently on the extremities, buttocks, perineum, or axilla, although they may occur anywhere including within the oral cavity. Although occasionally reported in infections with other organisms including Escherichia coli and Candida spp., these lesions are highly suggestive of Pseudomonas infection. However, only a minority of bacteremic infections develop this lesion. Other skin lesions encountered include vesiculopustular or maculopapular lesions and cellulitis. The diagnosis is made by the characteristic appearance of gram-negative microorganisms on Gram stain and recovery of P aeruginosa from blood or tissue cultures.
URINARY TRACT INFECTION
Urinary tract infection (UTI) with P aeruginosa occurs primarily in two settings: nosocomial infection or complicated urinary tract infection. Nosocomial infections involve patients with urinary catheterization, instrumentation, or surgery. Renal transplant recipients have a high risk of P aeruginosa urinary tract infection. Complicated urinary tract infections are often nosocomially acquired and occur in association with renal stones, chronic prostatitis, or urinary tract malformations. Rarely community-acquired P aeruginosa cystitis occurs in young patients as the result of transient colonization.
The clinical presentation of P aeruginosa UTI includes dysuria, increased frequency of micturition, and hematuria. Fever and flank pain occur in cases of pyelonephritis. Diagnosis is made by the recovery of P aeruginosa from cultures of a midstream or catheterized urine sample in association with pyuria or hematuria. Urinary tract infections contribute significantly to the total number of patients with P aeruginosa bacteremia and are associated with a better outcome than bacteremia associated with other sources.
Rare complications of Pseudomonas UTI include the sloughing of bladder mucosa associated with ulceration of the urinary bladder or renal infarcts caused by invasion of small blood vessels representing a form of ecthyma gangrenosum in the kidney. Treatment of UTI infection is complicated by the tenacity with which P aeruginosa adheres to urinary epithelium predisposing to chronic infection and relapse.
OPHTHALMOLOGIC INFECTION
P aeruginosa infections involving the eye take two predominant forms: keratitis and endophthalmitis. Keratitis often results from corneal ulceration induced by trauma. Risk factors include the use of contact lenses, especially extended wear soft contact lenses; topical ophthalmic steroid use; contaminated ophthalmic solutions; burn patients; prolonged coma; tracheostomy; ocular irradiation; or AIDS. In intensive care units, tracheal colonization, corneal drying, corneal abrasion, and a decrease in the bactericidal effect of lacrimal secretions contribute to the increased risk of infection.
Symptoms include pain, erythema, photophobia, purulent discharge, and blurred vision. On exam, a necrotic pale corneal ulcer is observed with adherent mucopurulent discharge and hypopyon formation (pus in the anterior chamber). Loss of vision may be rapid, and this condition is an ophthalmologic emergency necessitating prompt diagnosis and treatment. Diagnosis is made by ophthalmologic examination, scrapings from the ulcer, demonstration of organism presence on Gram stain, and organism recovery from culture. Treatment requires the application of antibiotic-containing ophthalmic solution combined with subconjunctival injection of antibiotics.
Endophthalmitis may result from keratitis, hematogenous spread, direct trauma, or surgery. The clinical signs and symptoms include a painful red eye with chemosis, hypopyon, anterior uveitis, decreased visual acuity, and in severe cases panophthalmitis. Therapy consists of combined topical, subconjunctival, intraocular and systemic treatment often combined with vitrectomy.
CENTRAL NERVOUS SYSTEM INFECTION
Infection of the central nervous system (CNS) with P aeruginosa occurs as a result of immunosuppression and altered local defenses. Spread to the CNS may be from a local source, such as malignant otitis externa or sinusitis, direct inoculation at the time of head trauma, or surgery (including the placement of external or internal shunts or dural grafts), or by hematogenous spread from a remote focus such as endocarditis. Infection may result in either meningitis or brain abscess.
Signs and symptoms of infection depend on whether bacteremia is associated with the infection. Bacteremic cases are usually acute with fever and signs of sepsis in addition to headache, nuchal rigidity, and photophobia characteristic of meningitis. Spinal fluid analysis demonstrates elevated protein and gram-negative bacteria on Gram stain, and in nonneutropenic patients an elevation in neutrophils is usual. Cases linked to direct inoculation resulting from head trauma, surgery, or neurosurgical procedure may occur with a more indolent course of fever, headache, and nonspecific signs of CNS infection. Spinal fluid analysis may establish the diagnosis. Cases complicated by abscess formation require aspiration or biopsy to confirm the diagnosis. Successful treatment requires correction of structural defects, removal of foreign materials, and drainage of abscesses when possible, in addition to antimicrobial treatment.
GASTROINTESTINAL INFECTIONS
The gastrointestinal tract is the principal portal of entry for Pseudomonas bacteremia in neutropenic patients receiving chemotherapy. In addition, these patients may develop localized gastrointestinal infection. Although infection may involve any part of the gastrointestinal tract, it particularly involves the cecum and rectum. Localized areas of necrosis and gangrene in the cecum are termed typhlitis. Pseudomonas infection of the cecum is characterized by hemorrhagic, necrotic ulcers with bacterial invasion of the submucosal blood vessels and an absence of inflammatory cells. Patients present with abdominal pain, and peritonitis may occur. The abdominal radiograph may reveal signs of perforation of a viscus. The rectum is also a source of bacterial abscesses in neutropenic patients, and P aeruginosa is most frequently the cause. Rectal abscesses may be the source of bacteremia or may result in localized spread causing gangrene. These lesions must be carefully searched for because the absence of inflammation in neutropenic patients may delay diagnosis. Rectal abscesses require prompt surgical drainage.
The second group of patients with ulcerative intestinal lesions due to P aeruginosa is young infants who develop necrotizing enterocolitis. Risk factors include prematurity, comorbid illness, and admission to the neonatal intensive care unit. Clinical signs are fever, irritability, vomiting, bloody diarrhea, dehydration, and abdominal distension. An abdominal radiograph may demonstrate pneumatosis intestinalis, portal air, or free peritoneal air.
Infection in Patients With Aids
Other Pseudomonas Species of Medical Importance
Table 1. Clues to the diagnosis of P aeruginosa.
Morphology and staining properties
- Gram-negative rod, straight or slightly curved, nonsporulating
- Motile with single polar flagellum
- Occurs singly, in pairs or short chains
Cultivation
- Grows readily on most common media
- Obligate aerobe
- Optimal culture at 37°C
- Nutritionally versatile and organic growth factors not required
Distinguishing microbiological characteristics
- Carbohydrate fermentation; negative
- Sugar oxidation; positive (glucose xylose); negative (maltose)
- Indophenol oxidase; positive
- Simmons citrate; positive
- L-Argenine dehydrolase; positive
- L-Lysine decarboxylase; negative
- L-Ornithine decarboxylase; negative
- Gas production from nitrate
- Positive hydrogen sulfide production in Kliger iron agar
- Positive growth at 42°C in brain heart infusion
Other microbiological tests
- Serology not routinely used in diagnosis (used in the diagnosis of P pseudomallei and P mallei)
- Serotyping and nucleic acid techniques have epidemiologic applications (eg, the investigation of nosocomial epidemics)
BOX 1. P aeruginosa Clinical Syndromes
Syndrome
Major Clinical Syndromes
Other Clinical Syndromes
Pulmonary Infections
- Primary pneumonia
- Ventilator-associated pneumonia
- Bacteremic pneumonia
Infections in Patients with Cystic Fibrosis
- Pneumonia
- Bronchitis
Bacteremia
- Bacteremia in association with neutropenia or AIDS
- Nosocomial bacteremia
Soft-Tissue Infections
- Ecthyma gangrenosum
- Burn wound sepsis
- Hot tub folliculitis
- Web space infection of the toe
- Green nail syndrome
Urinary-Tract Infection
- Catheter-associated UTI
- Complicated UTI
Ear Infections
- Otitis externa (swimmer’s ear)
- Malignant otitis externa
- Chronic suppurative otitis media
- Auricular perichondritis associated with ear piercing
Orthopedic Infections
- Prosthetic joint infection
- Post-traumatic open fracture osteomyelitis
- Puncture wound osteomyelitis of the foot
- Vertebral osteomyelitis
- Diabetic foot infection
- Chronic osteomyelitis
- Sternoarticular osteomyelitis
- Symphysis pubis osteomyelitis
Endocarditis
- Endocarditis in intravenous drug addicts
- Prosthetic valve endocarditis
Ophthalmologic Infection
- Keratitis
- Endophthalmitis
Central Nervous System Infections
- Meningitis/brain abscess associated with post-traumatic open skull fracture or postoperative neurosurgical procedure
- Meningitis in neonates or neutropenic patients
- Brain abscess associated with endocarditis
- Meningitis/brain abscess associated with contiguous suppurative focus
Gastrointestinal Infections
- Rectal abscess, typhlitis in neutropenic patients
- Necrotizing enterocolitis in infants
AIDS
- Pneumonia, bacteremia, and sinusitis
- Meningitis, osteomyelitis, malignant otitis externa
BOX 2. Treatment of P aeruginosa Clinical Syndromes
Syndrome
First Choice
Alternative Choice
Comments (Including Choice for Penicillin-Allergic Patients)
Pneumonia
EITHER
- Ceftazidime, 1-2 g (50 mg/kg) every 8 h
OR
- Cefepime, 1-2 g (50 mg/kg) every 12 h IV
OR
- Piperacillin, ticarcillin, or mezlocillin, 3 g (75 mg/kg) every 4 h IV
OR
- Ciprofloxacin, 400 mg (5-10 mg/kg) every 12 h IV or 500-750 mg (7.5-15 mg/kg every 12 h orally
OR
- Imipenem-cilastatin, 0.5 g (12.5 mg/kg) every 6 h IV, or meropenem, 1 g (40 mg/kg) every 8 h IV
OR
- Aztreonam, 2 g (30 mg/kg) every 6 h IV
PLUS
- Gentamicin or tobramycin, 2 mg/kg load, then 1.7 mg/kg every 8 h IV, or amikacin, 10 mg/kg load and 7.5 mg/kg every 12 h IV
- Treat for 14-21 days
In selected patients, monotherapy with ceftazidime, cefepime, ciprofloxacin, imipenem-cilastatin, meropenem, or aztreonam
Treatment for at least 14-21 days
Penicillin allergic: aztreonam, ciprofloxacin, imipenem-cilastatin, or meropenem plus aminoglycoside
Antibiotic dose adjustment for patients with cystic fibrosis
Treatment for at least 14-21 days
Bacteremia
Same treatment as for pneumonia; treat for at least 2 weeks
Same treament with monotherapy as for pneumonia; treat for at least 2 weeks
Same as per pneumonia
Burn Wound Sepsis
Same treatment as for pneumonia; duration of treatment individualized
Same treatment as for pneumonia; duration of therapy individualized
Beta-lactam allergic: see above
Avoid treating burn wound sepsis with monotherapy
Urinary Tract Infections
Upper tract infections: treat as per pneumonia with two agents for 2 weeks; may complete course with oral ciprofloxacin
Lower tract infection: ciprofloxacin, 500 mg (7.5 mg/kg) twice a day orally for 3-7 days
Upper tract disease: treat with monotherapy as per pneumonia for 2 weeks
Aztreonam, carbapenem, ciprofloxacin (same doses) IV for beta-lactam-allergic patient
Monotherapy usually suffices; if bacteremic, complicated upper tract disease, renal abscess formation, or neutropenic, treat as for bacteremia
Malignant Otitis Externa
Ceftazidime, cefepime, or ciprofloxacin (same doses as for pneumonia) for 4-8 weeks
Imipenem-cilastatin or -meropenem IV at same doses used for pneumonia for 4-8 weeks
Ciprofloxacin for beta-lactam-allergic patients
Orthopedic Infections
Same treatment as for pneumonia for 6 weeks
Single-agent therapy with ceftazidime, imipenem-cilastatin, or ciprofloxacin is less studied but may be a valid choice; doses as for pneumonia; treatment duration = 6 weeks
For penicillin-allergic patient, see above
Puncture wound osteomyelitis, ciprofloxacin, 500 mg (7.5 mg/kg) every 12 h orally for 4-6 weeks
Endocarditis
Ticarcillin, mezlocillin, piperacillin, ceftazidime, or cefepime
PLUS
Tobramycin or gentamicin, 8-12 mg/kg/d. Peak tobramycin or gentamicin conc in serum, 12-20 µg/ml; treatment for at least 6 weeks
Carbapenem, aztreonam, or ciprofloxacin, combined with aminoglycosides, is the alternative, but limited experience and optimal dosage require clarification
Aztreonam, carbapenem, or ciprofloxacin, and aminoglycoside combination for beta-lactam-allergic patient
Ciprofloxacin, 500 mg (7.5 mg/kg) every 12 orally, may be useful for long-term suppression of prosthetic valve endocarditis
Ophthalmic Infections
Keratitis: Gentamicin ophthalmic solution, 8 mg/ml every 30-60 min, plus subconjunctival gentamicin, 20 mg for first 3 days; total duration of therapy, = 1 week. Same doses for pediatric patients
Ophthalmic solution of enoxacin
Endophthalmitis: Combine parenteral and subconjuntival antipseudomonal penicillin and aminoglycoside with ophthalmic gentamicin and intraophthalmic gentamicin
Alternative: Ceftazidime or cefepime by parenteral, subconjunctival, and intraoccular routes
Duration: Intraoccular antibiotics for 7 days, other antibiotics till signs of infection resolve exact duration undetermined.
Central Nervous System Infections
Ceftazidime or cefepime, 50-100 mg/kg (same doses for pediatric patients) up to 2 g every 6 h or anti-pseudomonal penicillin (see above), meropenem, aztreonam, or ciprofloxacin plus aminoglycoside or ciprofloxacin parenterally ± intrathecally; treatment duration at least 2 weeks
Beta-lactam-allergic patient; see above. Imipenem-cilastatin should not be used because of risk of seizures
Gastrointestinal Infections
Same anitbiotics and doses as for pneumonia
Same antibiotics and doses as for pneumonia
Same antibiotics for penicillin-allergic patient as for pneumonia
Necrotizing enterocolitis (ticarcillin, piperacillin, or mezlocillin, 75 mg/kg every 6-8 h
PLUS
Tobramycin or gentamicin, 5-7 mg/kg/d)
Consult specialist text for dosages in low-birth-weight infants
Pediatric doses are given in parentheses.



