A decision to initiate antiretroviral therapy in an HIV-infected individual generally is based on the clinical and immunologic status of the patient and the risk of disease progression and should be guided by the patient’s plasma HIV-1 RNA level and CD4+ T-cell count. Most experts recommend that antiretroviral therapy be initiated in all HIV-infected adults with advanced HIV disease.
This includes those who are symptomatic with AIDS or severe symptoms, regardless of CD4+ T-cell counts and plasma HIV-1 RNA levels, as well as asymptomatic AIDS patients who have CD4+ T-cell counts less than 200/mm3, regardless of plasma HIV-1 RNA levels. Many experts also recommend that antiretroviral therapy be initiated in all patients diagnosed with acute HIV infection.The optimal time to initiate antiretroviral therapy in asymptomatic adults with CD4+ T-cell counts exceeding 200/mm3, especially those with relatively low plasma HIV-1 RNA levels, is controversial and remains to be determined.
Recommendations for Initial Antiretroviral Regimens
Antiretroviral therapy in HIV-infected adults who are treatment naive (have not previously received antiretroviral therapy) should be initiated with a potent multiple-drug regimen. Treatment should be aggressive with the goal of maximal suppression of viral load to undetectable levels.
Based on clinical data and expert opinion, the Panel on Clinical Practices for Treatment of HIV Infection recommends that antiretroviral therapy in treatment-naive adults be initiated with one of several multiple-drug regimens. These regimens can be classified as NNRTI-based regimens (1 NNRTI and 2 NRTIs), HIV protease inhibitor-based regimens (1 or 2 HIV protease inhibitors and 2 NRTIs), and triple NRTI regimens (3 NRTIs). Only regimens for which there are adequate clinical trial data to support their use (i.e., evidence of potency in an adequate randomized, prospective clinical study, assessment of tolerability, drug toxicity, drug interactions, pill burden, dosing frequency, food requirements) are included in Table 1.3 Data are insufficient to date to recommend other multiple-drug regimens for initial antiretroviral therapy, including triple class regimens (1 NRTI, 1 NNRTI, and 1 HIV protease inhibitor), NRTI-sparing regimens, regimens containing 5 or more antiretroviral agents, or other novel regimens.
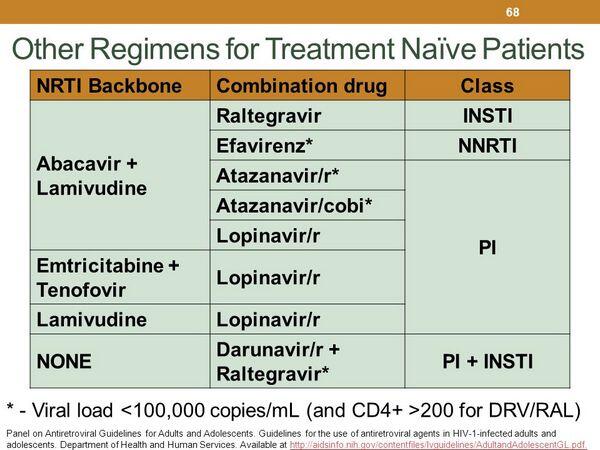
Regimens in Table 1 are designated as preferred for use in treatment-naive patients when clinical trial data suggest optimal efficacy and durability with acceptable tolerability and ease of use; regimens are designated as alternative when clinical trial data show efficacy but there are disadvantages compared to preferred regimens in terms of antiviral activity, demonstrated durable effect, tolerability, or ease of use. Based on individual patient characteristics, in some cases a regimen designated as alternative in the table may actually be a preferred regimen for a selected patient.
Advantages of NNRTI-based regimens include well documented virologic and immunologic efficacy; easier to use and adhere to than HIV protease inhibitor-based regimens; and delays use of HIV protease inhibitors. Advantages of HIV protease inhibitor-based regimens include well documented clinical, virologic, and immunologic efficacy; retroviral replication is targeted at 2 different steps; resistance to these regimens requires multiple mutations; and avoids adverse effects reported with NNRTIs. Advantages of triple NRTI regimens include deferring use of NNRTIs and HIV protease inhibitors and their associated adverse effects, minimal drug interactions, easy of use, and lack of cross-resistance to all NRTIs if the initial regimen fails. However, virologic efficacy of triple NRTI regimens is inferior to efavirenz-containing regimens and the Panel recommends that these regimens be reserved for use as an alternative to NNRTI-based or HIV protease inhibitor-based regimens when there are toxicity, drug interaction, or adherence concerns with the other regimens. In addition, based on evidence of a high rate of early virologic failure, a triple NRTI regimen of abacavir and tenofovir and lamivudine or a triple NRTI regimen of didanosine and tenofovir and lamivudine should not be used as the sole antiretroviral regimen at any time.
Recommended Pairings of NRTIs
Use of 2 NRTIs is recommended in all preferred and alternative NNRTI-based and HIV protease inhibitor-based regimens. These duel NRTI combinations are commonly used as the “backbone” of antiretroviral regimens; addition of a third or fourth agent confers sufficient potency for long-term efficacy. There currently are 7 commercially available NRTIs; in addition, tenofovir (a nucleotide reverse transcriptase inhibitor) is grouped with the NRTIs for purposes of therapeutic decisions. The choice of the specific NRTI combinations is based on potency, short- and long-term toxicity, drug interactions, propensity to select resistance mutations, and dosing convenience.
The combination of lamivudine and zidovudine is the NRTI pairing of choice for use in 3- or 4-drug multiple-drug regimens because of acceptable toxicity profiles, convenient dosing regimens, and extensive clinical experience. A fixed-combination preparation containing zidovudine and lamivudine (Combivir®) is commercially available for twice daily administration and can be used to decrease pill burden. The pairing of lamivudine and stavudine also is widely used but is more frequently associated with lipoatrophy, dyslipidemia, and mitochondrial toxicities. Lamivudine is recommended in preferred and alternative NRTI pairings because of low toxicity and favorable resistance patterns. Adverse effects reported with zidovudine (bone marrow suppression) or stavudine (lipoatrophy, peripheral neuropathy, lactic acidosis) may make it necessary to closely monitor for toxicities or use alternative NRTIs for selected patients.
Preferred alternative NRTI pairings are lamivudine and tenofovir or lamivudine and didanosine when used in conjunction with efavirenz. Although additional study is needed to determine long-term virologic efficacy, emtricitabine appears to have efficacy similar to lamivudine and may be used as an alternative to lamivudine in preferred or preferred alternative NRTI pairings.
NRTI combinations that should not be used include stavudine and zidovudine (possible in vitro and in vivo antagonism); emtricitabine and lamivudine (similar resistance profiles with potentially little additional virologic benefit); or stavudine and didanosine (toxicity, especially in pregnant women). Zalcitabine is rarely recommended because it is more toxic and less convenient (requires 3-times daily dosing) than other NRTIs. Therefore, regimens of zalcitabine and zidovudine (modest antiretroviral activity), zalcitabine and didanosine (overlapping toxicities), zalcitabine and lamivudine (evidence of in vitro antagonism), or zalcitabine and stavudine (overlapping toxicities) are not recommended.
Data are insufficient to date to determine the role of emtricitabine in dual NRTI combinations.
Regimens not Recommended
Monotherapy with any single antiretroviral agent is not considered an option for the treatment of HIV infection. No antiretroviral agent currently available, including HIV protease inhibitors, is capable of providing durable suppression of HIV replication when administered alone. In addition, monotherapy generally has been associated with rapid development of drug resistance and the potential for cross-resistance with related antiretroviral agents. Although zidovudine may be used alone in certain situations for perinatal prophylaxis, combination antiretroviral therapy should be initiated immediately after delivery or may be started during pregnancy as indicated. (See Guidelines for Use of Antiretroviral Agents: Antiretrovirals for Prevention of Maternal-fetal Transmission of HIV.)
Regimens that include 2 NRTIs alone (without a third antiretroviral agent) are considered suboptimal regimens and are no longer recommended. Although 2-drug regimen that included 2 NRTIs have been used for initial antiretroviral therapy in treatment-naive individuals and these regimens may be associated with initial declines in plasma HIV-1 RNA levels, they are less effective in providing durable suppression of HIV replication than 3-drug regimens that also include an antiretroviral agent from another class.
Triple NRTI regimens of abacavir, tenofovir, and lamivudine or didanosine, tenofovir, and lamivudine are not recommended for treatment naive patients or for previously treated patients because of a high rate of early virologic failure in clinical trials.
Didanosine and stavudine should be used concomitantly only when other NRTI pairings have failed or have caused unacceptable adverse effects and when the potential benefits outweigh the risk of toxicity. Concomitant use of didanosine and stavudine is associated with a high incidence of toxicity, especially peripheral neuropathy, pancreatitis, and lactic acidosis; several fatalities have been reported when this combination was used in HIV-infected pregnant women, apparently secondary to severe lactic acidosis with or without hepatic steatosis and pancreatitis.
Atazanavir should not be used concomitantly with indinavir. Both these HIV protease inhibitors can cause hyperbilirubinemia and jaundice and the possibility exists that these effects may be additive if both drugs are used concomitantly.
Although saquinavir hard gelatin capsules used in conjunction with low-dose ritonavir and 2 NRTIs is considered an alternative regimen for initial therapy, use of saquinavir hard gelatin capsules as the sole HIV protease inhibitor is not recommended because of the poor oral bioavailability reported with this formulation.
Use of hydroxyurea in antiretroviral regimens is not recommended. Although hydroxyurea may enhance the antiretroviral activity of didanosine, it also appears to enhance the toxicity of didanosine. In addition, hydroxyurea does not appear to increase CD4+ T-cell counts, presumably because of the drug’s cytotoxic effect.
Antiretroviral agents that are not recommended for initial therapy include delavirdine (only moderate antiviral activity compared with other NNRTIs), saquinavir (liquid-filled capsules) as sole HIV protease inhibitor (high pill burden), amprenavir as sole HIV protease inhibitor (high pill burden), ritonavir as the sole HIV protease inhibitor (GI toxicity), and the combination of nelfinavir and saquinavir (high pill burden).
Information Services (AIDSinfo) website (http://www.aidsinfo.nih.gov)