Dapsone (Aczone), a synthetic sulfone, is an antimycobacterial and antiprotozoal agent. It is also known as 4,4′-sulfonyldianiline, an antibiotic primarily used to treat leprosy (Hansen’s disease) and dermatitis herpetiformis, a skin condition characterized by blistering and itching. It is a white or creamy white, crystalline powder with a slightly bitter taste. The drug is very slightly soluble in water and freely soluble in alcohol.
Mechanism of Action
Dapsone is usually bacteriostatic in action. Its mechanism of action has not been fully elucidated. Because the antibacterial activity of the medicine is inhibited by p-aminobenzoic acid (PABA), the drug probably has a mechanism of action similar to that of sulfonamides, which involves the inhibition of folic acid synthesis in susceptible organisms. Some studies indicate that Dapsone may inhibit the alternate pathway of complement activation and interfere with the myeloperoxidase-H2O2-halide-mediated cytotoxic system within neutrophils. In vitro studies indicate that this medication stimulates neutrophil motility.
The drug also appears to inhibit spontaneous and induced synthesis of prostaglandin E2 by polymorphonuclear leukocytes obtained from healthy individuals or patients with leprosy. The mechanism of action in treating dermatitis herpetiformis is unknown; however, the drug only suppresses the disease, and cutaneous IgA and complement deposition are not affected by the drug. It has been suggested that the drug may act as an immunomodulator when used to treat dermatitis herpetiformis and other dermatologic diseases.
Pharmacokinetics
Dapsone is nearly completely absorbed after oral administration, with peak serum concentrations reached within 2-8 hours. Steady-state levels average 2.3 mcg/mL after 8 days of 200 mg daily dosing. Following a single 100 mg dose, serum levels range from 0.4-1.2 mcg/mL after 24 hours, with trace amounts detectable for up to 35 days post-discontinuation.
The volume of distribution is 1.5-2.5 L/kg, and Dapsone is retained in the skin, muscle, kidneys, and liver for up to 3 weeks after stopping therapy. It crosses the placenta and is distributed into breast milk, with concentrations reported at 1.1 mcg/mL in milk and 1.6 mcg/mL in maternal serum.
The medicine has a variable plasma half-life of 10-83 hours, averaging 20-30 hours. It is primarily metabolized to monoacetyldapsone (MADDS) in the liver, which can cause methemoglobinemia and hemolysis. About 20% of the drug is excreted unchanged in urine, while 70-85% is excreted as metabolites. Activated charcoal and hemodialysis can enhance the elimination of the medicine and its metabolites.
Dapsone (Aczone): Uses
The medicine works as an antibacterial agent by inhibiting the synthesis of dihydrofolic acid in bacteria, similar to sulfonamides. It is also recognized for its anti-inflammatory properties, which help reduce tissue damage during inflammatory responses. Dapsone can be administered orally or topically and is often prescribed with other medications for enhanced efficacy.
Leprosy
The drug is used in rifampin-based multiple-drug regimens for the treatment of multibacillary and paucibacillary leprosy. Although the medicine was used alone in the past for treating leprosy, the World Health Organization (WHO) and most clinicians currently recommend that rifampin-based multiple-drug regimens be used for treating all forms of leprosy.
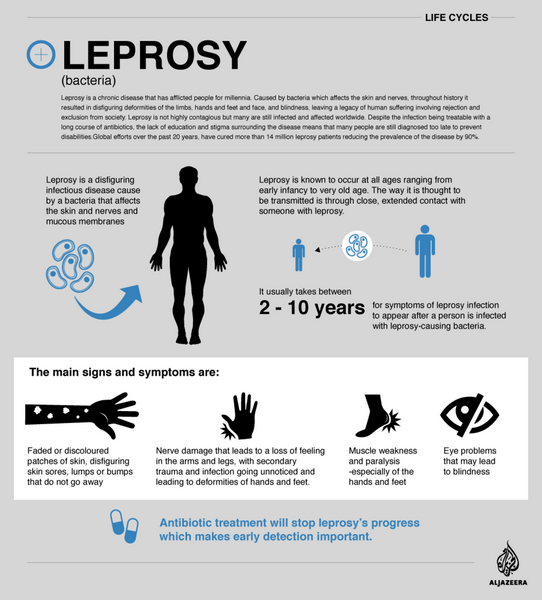
Multiple-drug regimens may reduce the infectiousness of the patient more rapidly as well as delay or prevent the emergence of resistant organisms. Because rifampin is bactericidal against M. leprae, once-monthly administration of rifampin is the principal component of the currently recommended multiple-drug regimens; Dapsone and clofazimine are included in the regimens to prevent the emergence of rifampin-resistant M. leprae.
Dermatitis Herpetiformis
Dapsone is the drug of choice for the treatment of dermatitis herpetiformis. Most clinicians recommend that sulfapyridine be used in treating dermatitis herpetiformis only when this drug cannot be used. In responsive patients, initiation of therapy usually results in a prompt decrease in pruritus and control of skin lesions of dermatitis herpetiformis; however, this medicine has no effect on cutaneous IgA and complement deposition.
Discontinuance of Dapsone therapy generally results in rapid exacerbation of lesions and severe pruritus. Using a gluten-free diet in conjunction with the drug improves clinical symptoms. It lowers the maintenance dosage requirements in approximately 60% of patients.
Pneumocystis Jiroveci (Pneumocystis Carinii) Pneumonia
Dapsone is used in conjunction with trimethoprim for the treatment of initial episodes of Pneumocystis jiroveci (formerly Pneumocystis carinii) pneumonia (PCP) in adults with acquired immunodeficiency syndrome (AIDS); it is designated as an orphan drug by the US Food and Drug Administration (FDA) for use in this condition. A combination regimen of the drug (100 mg once daily) and trimethoprim (20 mg/kg daily in 4 divided doses) given for 21 days is effective for the treatment of initial episodes of PCP in patients with AIDS, achieving a clinical response rate of 93% in one study in patients with mild to moderately severe initial episodes of the disease.
Most patients exhibit clinical improvement within 6 days, and the combination of drugs generally appears to be well tolerated. Therapy with this drug and trimethoprim appears to be as effective as oral co-trimoxazole for the treatment of initial episodes of mild to moderately severe PCP. Still, it is better tolerated than co-trimoxazole in AIDS patients.
Prevention of PCP
The medicine is used alone or in conjunction with pyrimethamine for the prevention of PCP in HIV-infected individuals; Dapsone is designated as an orphan drug by the FDA for use in this condition. Although co-trimoxazole generally is the drug of choice for both primary and secondary prophylaxis of PCP in HIV-infected individuals, the Prevention of Opportunistic Infections Working Group of the US Public Health Service and the Infectious Diseases Society of America (USPHS/IDSA) and other clinicians recommend this drug or Dapsone and pyrimethamine (with leucovorin) as alternatives.
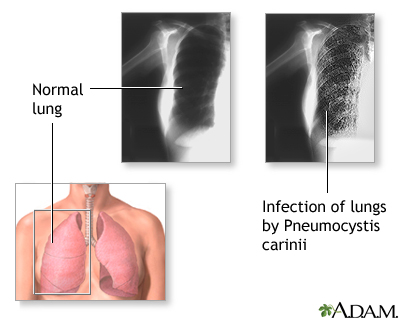
The medication alone is recommended as an alternative to co-trimoxazole for PCP prophylaxis in pregnant women.
The USPHS/IDSA currently recommends that children born to HIV-infected mothers receive primary prophylaxis against PCP beginning at 4-6 weeks of age; prophylaxis can be discontinued in children subsequently found not to be infected with HIV, but those whose HIV status remains unknown should continue to receive PCP primary prevention for the first year of life.
Prevention of Recurrence of PCP
The USPHS/IDSA currently recommends that HIV-infected individuals who have a history of PCP receive long-term suppressive or chronic maintenance therapy (secondary prevention) to prevent recurrence.
The same regimens recommended for primary PCP prophylaxis are used for secondary prevention. Secondary prevention is generally administered for life unless immune recovery has occurred due to potent antiretroviral therapy. Current evidence indicates that secondary prophylaxis against PCP can be discontinued in HIV-infected adults and adolescents responding to potent antiretroviral therapy who have a sustained (3 months or longer) increase in CD4+ T-cell counts from less than 200/mm3 to greater than 200/mm3.
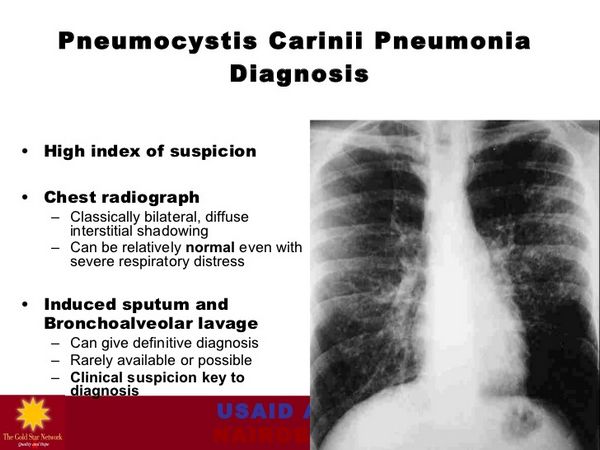
The USPHS/IDSA states that children who have a history of PCP should receive lifelong suppressive therapy to prevent recurrence. The safety of discontinuing secondary PCP prophylaxis in HIV-infected children has not been extensively studied.
Toxoplasmosis Prevention
Although Dapsone is not used for the treatment of toxoplasmosis, a 2-drug regimen of this drug and pyrimethamine is recommended as an alternative to co-trimoxazole for primary or secondary prevention of Toxoplasma gondii encephalitis and the drug alone is recommended as an alternative to co-trimoxazole for secondary toxoplasmosis prophylaxis.
The USPHS/IDSA currently recommends primary prophylaxis against T. gondii encephalitis for all HIV-infected adults and adolescents who are seropositive for Toxoplasma IgG antibody and have CD4+ T-cell counts less than 100/mm3. HIV-infected infants and children with severe immunosuppression who are seropositive for Toxoplasma IgG antibody also should receive primary prophylaxis against T. gondii encephalitis. The USPHS/IDSA recommends co-trimoxazole as the drug of choice for primary prophylaxis against toxoplasmosis in HIV-infected adults, adolescents, and children.
A regimen of Dapsone and pyrimethamine (with leucovorin) is the recommended alternative for primary prophylaxis against toxoplasmosis in patients who cannot tolerate co-trimoxazole.
Toxoplasmosis
Current evidence indicates that primary toxoplasmosis prophylaxis can be discontinued with minimal risk of developing toxoplasmic encephalitis in adults and adolescents responding to potent antiretroviral therapy who have a sustained (3 months or longer) increase in CD4+ T-cell counts from less than 200/mm3 to greater than 200/mm3.
The USPHS/IDSA states that discontinuance of primary toxoplasmosis prophylaxis is recommended in HIV-infected adults and adolescents who have a sustained (3 months or longer) increase in CD4+ T-cell counts to greater than 200/mm3 because prophylaxis appears to add little benefit in terms of disease prevention for toxoplasmosis. Discontinuance reduces the medication burden, the potential for toxicity, drug interactions, selection of drug-resistant pathogens, and cost. If primary toxoplasmosis prophylaxis is discontinued in adults and adolescents meeting the recommended criteria, the USPHS/IDSA states it should be restarted if the CD4+ T-cell count decreases to less than 100-200/mm3.
The safety of discontinuing primary prophylaxis in HIV-infected children receiving potent antiretroviral therapy has not been extensively studied.
This medication is not recommended for secondary toxoplasmosis prophylaxis.
Other Uses
Dapsone has effectively treated bullous eruptions or mucocutaneous lesions in patients with systemic lupus erythematosus and discoid lupus erythematosus resistant to conventional antimalarial or corticosteroid therapy.
The medicine has also been used successfully in treating other diseases characterized by bullous eruptions, such as bullous pemphigoid, pemphigus vulgaris, and Hailey-Hailey disease.
The drug has been used in a limited number of patients in the treatment of several inflammatory dermatoses (e.g., pyoderma gangrenosum, erythema elevatum diutinum, Weber-Christian disease, Sweet’s syndrome, polyarteritis nodosa, granuloma faciale) and pustular dermatoses (e.g., herpes gestationis, impetigo herpetiformis, pustular psoriasis, follicular mucinosa). Dapsone has been used with some success in the treatment of rheumatoid arthritis, relapsing polychondritis, and allergic vasculitis.
Administration and Dosage
The drug is administered orally. For administration to children, commercially available tablets of Dapsone have been crushed and dissolved in strawberry syrup; however, studies evaluating the bioavailability of the drug following administration of this preparation have not been published to date.
|
Condition |
Age Group |
Dapsone Dosage |
Additional Medications |
Duration |
|
Multibacillary Leprosy |
Adults |
100 mg daily |
Rifampin (600 mg once monthly), Clofazimine (50 mg daily, 300 mg once monthly) |
12 months |
|
Children (10-14 years) |
50 mg daily |
Rifampin (450 mg once monthly), Clofazimine (50 mg every second day, 150 mg once monthly) |
12 months |
|
|
Children (<10 years) |
25 mg daily (adjusted based on weight) |
Rifampin (300 mg once monthly), Clofazimine (50 mg twice weekly, 100 mg once monthly) |
12 months |
|
|
Note: Supervised administration recommended for some doses |
||||
|
Additional therapy for high bacteriologic index patients with no improvement after initial treatment |
Additional 12 months if needed |
|||
|
Paucibacillary Leprosy |
Adults |
100 mg daily |
Rifampin (600 mg once monthly) |
6 months |
|
Children (10-14 years) |
50 mg daily |
Rifampin (450 mg once monthly) |
6 months |
|
|
Children (<10 years) |
25 mg daily (adjusted based on weight) |
Rifampin (300 mg once monthly) |
6 months |
|
|
Dapsone may be discontinued if severe adverse effects occur; clofazimine can be substituted |
||||
|
Dermatitis Herpetiformis |
Adults |
Start with 50 mg daily; may increase to 300 mg daily. Maintenance dosage generally ranges from 25-400 mg daily. |
Long-term |
|
|
Children |
Dosage adjusted based on weight; typically lower than adult dosage |
Long-term |
||
|
Pneumocystis jiroveci Pneumonia Treatment |
Adults |
100 mg once daily |
Trimethoprim (5 mg/kg three times daily) |
21 days |
|
Pneumocystis jiroveci Pneumonia Prevention |
Adults |
50 mg twice daily or 100 mg once daily |
Can also be given with pyrimethamine and leucovorin in various regimens |
Long-term |
|
Children (>1 month) |
2 mg/kg (max. 100 mg) once daily or 4 mg/kg (max. 200 mg) once weekly |
Long-term |
||
|
Toxoplasmosis Prevention |
Adults |
50 mg once daily or 200 mg once weekly |
Pyrimethamine (50/75 mg weekly), oral leucovorin (25 mg weekly) |
Long-term |
|
Children (>1 month) |
2 mg/kg or 15 mg/m² (max. 25 mg) once daily |
Pyrimethamine (1 mg/kg once daily), oral leucovorin (5 mg every three days) |
Long-term |
The medicine may be discontinued if severe adverse effects occur; in some cases, clofazimine can be substituted.
Side Effects
This medication is associated with a range of side effects, some of which can be serious. Common side effects include gastrointestinal disturbances such as nausea and abdominal pain, as well as hematologic issues like hemolytic anemia and agranulocytosis, particularly in individuals with glucose-6-phosphate dehydrogenase deficiency. Additionally, neurological effects such as peripheral neuropathy and mood changes have been reported, necessitating careful monitoring during treatment.
Hematologic Effects
The most frequent adverse effects of Dapsone are dose-related hemolytic anemia and methemoglobinemia. Hemolysis occurs in most patients receiving 200 mg or more of this medication daily; however, symptomatic anemia occurs only occasionally. The manufacturer states that the hemoglobin level generally decreases by 1-2 g/dL, the reticulocyte count is increased by 2-12%, erythrocyte life span is shortened, and methemoglobinemia occurs in most patients receiving this drug. Heinz body formation also occurs frequently. Unless severe, hemolysis or methemoglobinemia does not generally require discontinuance of the therapy.
Generally, Dapsone is well tolerated in AIDS patients. However, asymptomatic methemoglobinemia has been reported in two-thirds of such patients receiving 100 mg of the drug daily concomitantly with trimethoprim 20 mg/kg daily.
Prophylactic administration of ascorbic acid, folate, and iron reportedly may prevent some of the adverse hematologic effects of the medicine. Leukopenia has been reported occasionally during therapy, and potentially fatal agranulocytosis and aplastic anemia have been reported rarely.
Leprosy Reactional States
Effective therapy for leprosy with this medication or other agents often leads to significant clinical changes known as leprosy reactional states, classified into two types: reversal reactions (type 1) and erythema nodosum leprosum (ENL) reactions (type 2). Reversal reactions primarily affect patients with borderline or tuberculoid leprosy and result from an enhanced hypersensitivity response, causing swelling, erythema, fever, and potential nerve damage. ENL, occurring mainly in multibacillary leprosy patients, is less common with current multidrug regimens that include clofazimine compared to Dapsone monotherapy.
Treatment depends on severity; severe cases may require hospitalization. Standard antileprosy regimens typically continue, with corticosteroids used for nerve injury or ulceration. ENL is treated with analgesics, corticosteroids, thalidomide, and clofazimine. Early diagnosis and management are crucial to reduce morbidity associated with these reactions, and treatment should involve an expert in leprosy care.
Dermatologic Reactions
Adverse cutaneous effects, which usually result from sensitization to Dapsone, occur rarely during therapy with the drug. Cutaneous reactions include exfoliative dermatitis, toxic erythema, multiforme, epidermal necrolysis, morbilliform and scarlatiniform eruptions, urticaria, and erythema nodosum. If a new or toxic dermatologic reaction occurs during therapy, the drug should be discontinued and appropriate therapy initiated. Rash reportedly occurs in about 30-40% of AIDS patients receiving this drug concomitantly with trimethoprim but less frequently in those receiving the medication alone; despite such rash, a substantial proportion of patients who do not tolerate co-trimoxazole can tolerate the medicine.
Nervous System Effects
Peripheral neuropathy with motor loss has been reported rarely in patients receiving high dosages of Dapsone (200-500 mg daily). If muscle weakness occurs during therapy, the drug should be discontinued; complete recovery may occur if the drug is withdrawn, but may take many months to several years. The mechanism of recovery is reportedly by axonal regeneration, and some recovered patients have tolerated retreatment with the drug using a lower dosage.
Although peripheral neuropathy has not been reported to date in patients with leprosy receiving this medication, presumably because a lower dosage is used, this adverse effect may be complex to distinguish from a leprosy reactional state. Insomnia, headache, nervousness, vertigo, and psychosis have also been reported with this medication.
GI Effects
Adverse GI effects, including anorexia, abdominal pain, nausea, and vomiting, have occurred in patients receiving Dapsone.
Hepatic Effects
Toxic hepatitis and cholestatic jaundice have been reported with this drug. Cholestatic jaundice may be a hypersensitivity reaction and generally appears to be reversible following discontinuance of the drug. Adverse hepatic effects have occurred shortly after initiation of the therapy. They may be manifested by increased serum concentrations of alkaline phosphatase, AST (SGOT), bilirubin, and LDH. Liver function test abnormalities reportedly occur more frequently during combined Dapsone and trimethoprim therapy than during the drug alone. Hyperbilirubinemia has also occurred during therapy and may occur more often in patients with G-6-PD deficiency.
Renal and Electrolyte Effects
Albuminuria, nephrotic syndrome, and renal papillary necrosis have occurred rarely during therapy. Mild, generally asymptomatic hyperkalemia has been reported frequently in patients receiving combined Dapsone and trimethoprim therapy. Still, serum potassium concentrations generally returned to normal during continued therapy.
Other Adverse Effects
Blurred vision, tinnitus, fever, phototoxicity, hyperpigmented macules, hypoalbuminemia without proteinuria, drug-induced lupus erythematosus, and an infectious mononucleosis-like syndrome have been reported with this drug. Tachycardia has also occurred, particularly with excessive dosage of the drug.
Precautions and Contraindications
The drug is contraindicated in patients who are hypersensitive to the drug or its derivatives, such as sulfoxone sodium. It should not be administered to patients with severe anemia; the anemia should be treated before initiation of therapy.
The drug should be used cautiously in patients with G-6-PD deficiency, methemoglobin reductase deficiency, or hemoglobin M.
The medicine should also be used in patients exposed to other drugs or agents capable of inducing hemolysis and in patients with conditions associated with hemolysis (e.g., certain infections and diabetic ketosis).
Some clinicians recommend that screening for G-6-PD deficiency be performed before initiating therapy in human immunodeficiency virus (HIV)-infected patients and that hemoglobin and methemoglobin concentrations and hematocrit be monitored periodically in such patients, particularly those receiving the drug concomitantly with trimethoprim. Complete blood cell counts (CBCs) should be performed frequently during dapsone therapy.
Some clinicians recommend that CBCs be performed weekly during the first month of therapy, monthly for the next 6 months, and every 6 months after that. If a substantial reduction in leukocytes, platelets, or hematopoiesis is evident, Dapsone should be discontinued and the patient closely monitored. Because toxic hepatitis and cholestatic jaundice have been reported, liver function should be monitored, when feasible, before and during therapy with the drug. If any abnormality in liver function is evident, the drug should be discontinued until the source of the abnormality is established.
Patients should be instructed to report to their clinician the presence of sore throat, fever, pallor, purpura, or jaundice during therapy.
Mutagenicity and Carcinogenicity
Dapsone was not mutagenic in microbial tests using Salmonella typhimurium, with or without microsomal activation. It has been found to be carcinogenic in animal studies. The drug has caused mesenchymal tumors in the spleen and peritoneum of male rats and female mice and thyroid carcinoma in female rats.
Pregnancy, Fertility and Lactation
Animal reproduction studies have not been performed with this medicine. Although it has been used in pregnant women without evidence of fetal abnormalities, the drug should be used during pregnancy only when clearly needed. In patients with leprosy, some clinicians consider that the benefits of maintaining therapy during pregnancy outweigh the potential risks to the fetus. Infertility has been reported in males receiving the medication; in 2 patients, fertility was restored following discontinuance of the drug.
Because the drug is distributed into milk and because of the tumorigenic potential demonstrated in animal studies, it should not be used in nursing women. A decision should be made whether to discontinue nursing or the drug, taking into account the importance of the drug to the woman.
Drug Interactions
Dapsone may interact with various medications, leading to increased risks of adverse effects.
|
Drug |
Interaction Type |
Effects |
Recommendations |
|
Didanosine (ddI) |
Decreased efficacy of dapsone |
Failure to prevent Pneumocystis jiroveci pneumonia in ~40% of patients |
Administer dapsone at least 2 hours apart from didanosine; ongoing studies to clarify interaction needed. |
|
Clofazimine |
Minimal effect on dapsone pharmacokinetics |
Transient increase in urinary excretion; potential reduction in clofazimine’s anti-inflammatory effects |
Continue both medications; further studies recommended to confirm interactions. |
|
Pyrimethamine |
Increased risk of hematologic adverse effects |
Agranulocytosis reported during concomitant use |
Monitor patients more frequently for hematologic effects; use with caution in G-6-PD deficiency patients. |
|
Rifampin |
Decreased serum concentrations of dapsone |
Serum levels may be 7-10 times lower when used together |
Generally, no dosage change required during concomitant therapy for leprosy. |
|
Trimethoprim |
Increased plasma concentrations of dapsone |
Enhanced efficacy for treating Pneumocystis pneumonia; higher rates of methemoglobinemia and discontinuation due to adverse effects |
Periodic monitoring for toxicity recommended; further pharmacokinetic studies needed. |
|
Probenecid |
Limited evidence of interaction |
Potential interference with urinary excretion of dapsone metabolites |
Considered unlikely and/or clinically irrelevant; no specific recommendations available. |
|
Other Hemolytic Agents |
Increased risk for methemoglobinemia and hemolysis |
Caution advised in patients with G-6-PD deficiency or exposed to hemolytic agents |
Monitor closely for hemolytic effects when used with other agents capable of inducing hemolysis. |
Overdose
Overdosage generally results in nausea, vomiting, and hyperexcitability within a few minutes to up to 24 hours later. Methemoglobin-induced depression, seizures, and severe cyanosis may occur and require prompt treatment. Hemolysis may occur 7-14 days after an acute ingestion.
In patients who do not have G-6-PD deficiency, dapsone-induced methemoglobinemia should be treated with methylene blue (1-2 mg/kg given by slow IV injection). The effect is generally complete within 30 minutes, but methylene blue may need to be readministered if methemoglobin reaccumulates.
Alternatively, in nonemergency situations, methylene blue may be given orally at 3-5 mg/kg every 4-6 hours. Methylene blue should not be administered to patients with G-6-PD deficiency since methylene blue reduction depends on G-6-PD. Orally administered activated charcoal (20 g 4 times daily) has been shown to substantially enhance the elimination of the drug and its monoacetyl derivative in several cases of acute overdosage. Some clinicians recommend it as a treatment of choice in the management of acute intoxication. Hemodialysis also enhances the elimination of the medicine and its monoacetyl derivative.
Storage
Dapsone tablets should be stored in well-closed, light-resistant containers at less than 40°C, preferably between 15-30°C.
Other Names of Dapsone (Aczone)
Dapsone Oral Tablets 25 mg, Dapsone Tablets, Jacobus 100 mg Dapsone Tablets, Jacobus, Acnesone Gel, Acnesone (Skin).

















