Clinical Findings
The spectrum of influenza infection ranges from subclinical cases to fulminating viral pneumonia.
Signs and Symptoms (Box 1)
There are no specific physical examination findings associated with influenza. The patient usually appears ill and has fever. A clear nasal discharge is common. A typical uncomplicated case of influenza illness begins abruptly and is manifested by sore throat, headache, fever, chills, myalgias, anorexia, and extreme fatigue. Fever is usually between 38 and 40 °C but may be higher and usually lasts for ~3 days (but = 5 days). Other respiratory tract manifestations include cough, which is usually nonproductive, and a runny or stuffy nose. Substernal tenderness, photophobia, abdominal pain, and diarrhea occur less frequently. Despite severe sore throat, the mucous membranes of the pharynx may be unremarkable or hyperemic without exudates. Small tender cervical lymph nodes may be palpable, and the lungs are usually clear, although scattered rhonchi and crackles can be heard in as many as a quarter of patients.
In the elderly, fever may be absent and the presenting signs may be anorexia, lassitude, confusion, and rhinitis. In children, fevers are often higher and can lead to febrile seizures. Gastrointestinal manifestations, such as vomiting, abdominal pain, and diarrhea, and other complications such as myositis, croup (tracheobronchitis), and otitis media also occur more frequently in children. Unexplained fever may be the primary manifestation in neonates.
Laboratory Findings
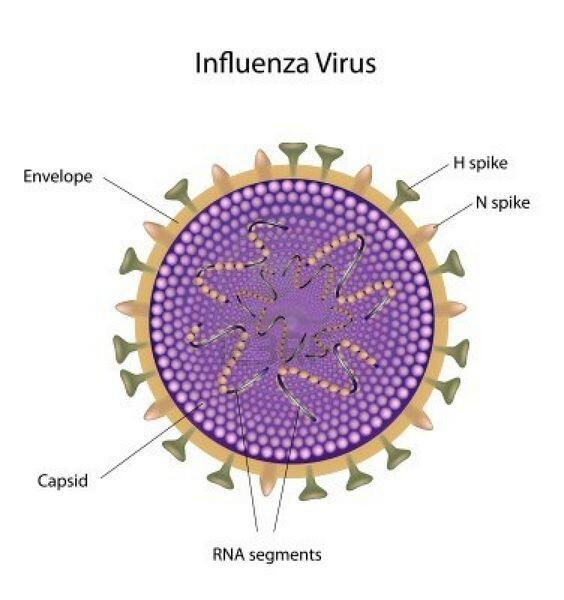
Results of routine laboratory tests are not specific for influenza. Leukocyte counts are variable. Severe leukopenia has been described in overwhelming illness. Leukocytosis of > 15,000 cells/mL should raise the suspicion of a secondary bacterial process. Specific laboratory tests to confirm influenza include viral culture, rapid antigen detection, and serology.
Virus can be isolated from nasal washing and nasopharyngeal swab specimens obtained within 3-4 days of illness. Virus is grown either in embryonated hens’ eggs or in primary tissue culture systems, such as Madin-Darby canine kidney cells. Viral culture offers specific information and the ability to further characterize the isolate, but the sensitivity of this technique is highly dependent on the timing of when the specimen is obtained. Results usually are not available for at least 3 days.
Rapid diagnostic techniques to identify viral antigens in clinical specimens include immunofluorescence, enzyme immunoassay, and time-resolved fluoroimmunoassay. Several rapid test kits are now commercially available that either can 1) detect influenza A but not influenza B virus; 2) detect influenza A or B virus but not distinguish between them; or 3) detect influenza A or B virus and distinguish between them. In general, these tests are more specific than sensitive but head-to-head comparison data are not available. These tests can yield results within 30 min. Reverse transcriptase polymerase chain reaction assays have also been used to detect influenza virus RNA in clinical specimens.
Serologic techniques for measuring antibody against influenza include hemagglutination inhibition, neutralization, enzyme immunoassay, and complement fixation. In general, serology is a sensitive technique for establishing influenza infections. However, serologic tests usually require acute and convalescent serum samples to demonstrate a significant increase in antibody level. The measurement of influenza antibody levels in a single-serum sample is rarely helpful. Ideally, acute and convalescent blood samples should be collected, respectively, within 2-3 days of illness and at 3 weeks after the start of illness. The most commonly used serologic test to document influenza virus infection is hemagglutination inhibition because it (and neutralization) is more sensitive than complement fixation and allows subtype and strain-specific antibody to be measured.
Imaging
A chest X-ray may show an infiltrate in those with primary viral pneumonia or a complicating bacterial pneumonia.
Differential Diagnosis
Other respiratory viruses, including respiratory syncytial virus, adenovirus, parainfluenza virus, and rhinovirus, as well as other organisms, such as Mycoplasma pneumoniae, can produce illness similar to influenza. However, outbreaks of febrile respiratory illness cases during the winter through spring months are characteristic of influenza. Information on local influenza activity is usually available from the local health department.
Complications
The most common serious complications of influenza include exacerbation of underlying chronic pulmonary and cardiopulmonary diseases, such as worsening of chronic obstructive pulmonary disease, asthma, and congestive heart failure, as well the development of pneumonia. Secondary bacterial pneumonias occur much more frequently than primary viral pneumonia and usually are associated with Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae. With complicating bacterial pneumonia, the patient typically reports a period of improvement followed by the appearance of signs and symptoms suggestive of pneumonia, such as pleuritic chest pain, productive cough, and fever. On chest radiography, lobar consolidation can be seen and sputum smears show polymorphonuclear leukocytes with bacteria.
Primary viral pneumonia is an infrequent but often fatal complication in which influenza progresses within the first 24-48 h of illness and leads to increasing dyspnea, tachypnea, and cyanosis. On presentation, fever and cough are usually present and the patient appears uncomfortable and dyspneic. On chest auscultation, diffuse fine rales with wheezes or coarse breath sounds may be evident. Sputum may be scanty but can be blood streaked. Chest roentgenograms usually show bilateral interstitial infiltrates or a picture consistent with acute respiratory distress syndrome. The Gram stain of the sputum often shows few polymorphonuclear cells or bacteria. Virologic cultures of the respiratory secretions often yield virus. The value of antiviral medications in this setting is unknown. Cases of viral pneumonia described in the 1918 and 1957 pandemics were associated with underlying cardiac valvular disease (frequently mitral stenosis from rheumatic heart disease) and pregnancy.
In addition to these pneumonias, mixed viral and bacterial pneumonias with features of both etiologies have been described. Other respiratory tract complications include bacterial sinusitis, croup, and otitis media.
Reye’s syndrome has been described primarily in children < 18 years of age. In almost all cases, Reye’s syndrome appears to be a complication resulting from the use of salicylates, most commonly aspirin, to treat certain viral illnesses. Reye’s syndrome usually presents several days after an unremarkable viral illness. The syndrome usually presents with nausea and vomiting followed by central nervous system (CNS) changes such as lethargy, delirium, seizures, or coma. Reye’s syndrome has been primarily seen in children treated with aspirin for influenza B virus and varicella-zoster virus infections but also for infections by influenza A virus. Since the 1980s, the incidence of Reye’s syndrome has decreased dramatically in the United States after warnings were issued regarding the link between the treatment of children with aspirin and this syndrome.
Myocarditis and pericarditis were reported in association with influenza during the 1918-1919 pandemic but have been documented infrequently since then. Minor electrocardiogram changes in the setting of influenza can often be seen in patients with underlying heart disease. Myositis with rhabdomyolysis and myoglobinuria has been reported but is uncommon. Toxic shock syndrome associated with secondary staphylococcal infection after acute influenza also has been reported. In addition, a number of CNS complications including encephalopathy, encephalitis, transverse myelitis, and Guillain-Barre syndrome have all been reported, but their association with influenza remains unclear.
Treatment
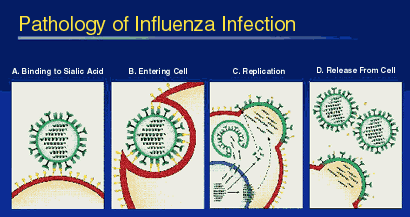
Uncomplicated cases of influenza are usually treated symptomatically. Salicylates should be avoided in children < 18 years of age because of the risk of Reye’s syndrome. There are two classes of licensed antiviral agents, the adamantines and the neuraminadase inhibitors, with specific activity against influenza viruses. The adamantines, amantadine hydrochloride and rimantadine hydrochloride, have specific activity against influenza A virus but not B virus. The neuraminadase inhibitor drugs, zanamivir and oseltamivir, have activity against both influenza A and B viruses. Amantadine is licensed for use in children and adults, whereas rimantadine is licensed for use in adults. Both drugs can be given for treatment of or prophylaxis against influenza A virus but are ineffective against influenza B virus. These chemically related drugs are equally effective and appear to inhibit viral replication by blocking the ion channel function of the viral M2 protein. Viral resistance to both compounds is associated with changes in the M2 protein. Although the rapid emergence of resistant viruses has been demonstrated both in vitro and in vivo, the risk of transmission of these resistant viruses remains unclear. Resistant viruses have been most often isolated from individuals receiving treatment and less often from contacts.
When administered prophylactically, both agents are ~70-90% effective in preventing illness caused by influenza A viruses. Subclinical infections still can occur while taking these drugs. Although chemoprophylaxis can be used alone in persons for whom vaccination is contraindicated, it is preferable to administer these agents as an adjunct to vaccination in high-risk groups. Since these drugs do not interfere with antibody response to vaccination, they can be used to provide prophylaxis to persons who were vaccinated but who have not yet had adequate time (usually 2 weeks) to mount a vaccine antibody response. To control an institutional influenza outbreak, antiviral agents are most effective when administered to all residents.
When administered within 48 h of illness onset, these agents have also been shown to reduce the severity and duration of influenza in young and healthy adults and children. Similar controlled studies have not been conducted among persons at high risk for complications.
The major pharmacological differences between these agents are their pharmacokinetic and side-effect profiles. More than 90% of amantadine is excreted unchanged by the kidneys, whereas ~75% of rimantadine is metabolized by the liver (however, unmetabolized rimantadine and its metabolites are renally excreted).
Both medications can lead to CNS and gastrointestinal side effects. In one study, the incidence of CNS side effects in young and healthy adults using 200 mg/d was higher among those taking amantadine (14%) than rimantadine (6%) or placebo (4%). CNS side effects include nervousness, anxiety, difficulty concentrating, and light-headedness. More serious side effects (eg, marked behavioral changes, delirium, hallucinations, agitation, and seizures) have been associated with high plasma drug concentrations, particularly among persons with renal insufficiency, seizure disorders, or certain psychiatric disorders, or among elderly persons who were taking amantadine at doses of 200 mg/d. Approximately 3% of those taking either drug develop gastrointestinal side effects, such as nausea and anorexia. Side effects cease soon after stopping the drug, and lower doses appear to be associated with a lower incidence of side effects.
The usual therapeutic and prophylactic dosage of amantadine and rimantadine in adults < 65 years is 100 mg orally twice a day (Box 2). Doses should be reduced in children younger than 10 years, adults 65 years and older, especially those in nursing homes, and persons with renal insufficiency or severe hepatic dysfunction (for rimantadine).
The neuraminadase inhibitors were approved in 1999 for treatment of uncomplicated influenza. Zanamivir is orally inhaled and was approved for treatment of persons > 7 years. Oseltamivir is orally administered and was approved for treatment of persons > 1 year. Similar to the adamantines, both neuraminadase inhibitors can be effective in reducing illness by approximately one day when used within 2 days of illness. Although only oseltamivir has been approved for chemoprophylaxis, recent community studies suggest that both zanamivir and oseltamivir are approximately 80% effective in reducing febrile influenza illness when administered as chemopropylaxis. Oseltamivir was approved in 2000 for chemoprophylaxis of persons > 13 years.
In placebo controlled studies of persons with uncomplicated influenza, persons receiving zanamivir or placebo reported similar rates of adverse events including diarrhea, nausea, sinusitis, nasal signs and symptoms, bronchitis, cough, headache, dizziness, and ear, nose throat infections. In patients with asthma or chronic obstructive pulmonary disease, more patients taking zanamivir than placebo had a > 20% decrease in forced expiratory volume in 1 second (FEV1) or peak expiratory flow. In addition, persons with underlying asthma or chronic obstructive pulmonary disease have been reported to experience respiratory deterioration following use of zanamivir. Caution should be exercised when prescribing zanamivir to patients with asthma or chronic obstructive pulmonary disease. Such patients should have a fast acting bronchodilator available when inhaling zanamivir. Oseltamivir has been associated with higher levels of nausea or vomiting (approximately 9-10%) than in persons taking placebo.
Resistance to the neuraminadase inhibitors can be induced in influenza A and B viruses in-vitro but there is little information to indicate the clinical significance of these findings. Available information suggests that resistance to these compounds develops less frequently than with the adamantines.
The recommended dosage of zanamivir is two inhalations (a total of 10 mg) twice daily about 12 hours apart for five days. The manufacturer does not recommend changes in dosage based on age or renal function. Inhaled zanamivir has a half-life of about 2.5-5.1 hours and is excreted unchanged in urine. The recommended dosage of oseltamivir for treatment in persons > 13 years or for younger children who weigh > 40 is 75 mg twice daily. In children < 13 years and who weigh < 40 kg the recommended dosages vary by weight: 30 mg twice daily for children < 15 kg; 45 mg twice daily for children > 15 kg to 23 kg; and 60 mg twice daily for children > 23 to 40 kg. Oseltamivir is approved for chemoprophylaxis in children > 13 years and the dosage is 75 mg once a day. There is no recommended change in dosage for elderly persons. However, in patients with renal dysfunction and a creatinine clearance between 10 and < 30, the recommended treatment dosage is 75 mg once a day and the recommended chemoprophylaxis dosage is 75 mg every other day. No recommendations are available for patients undergoing renal dialysis.
Ribavirin has been reported to have efficacy against influenza A and B infections when administered as an aerosol for treatment.
Prognosis
In most cases, illness from influenza resolves within a week, but cough and malaise may persist for several days to a few weeks longer. In a minority of patients, fatigue may persist for months.
Prevention & Control
Annual administration of influenza vaccine is the most effective approach for preventing illness caused by influenza. In the United States, the currently licensed vaccine is an inactivated vaccine (either killed whole virus or subunit preparations) that contains three contemporary circulating strains of influenza A (H1N1), influenza A (H3N2), and influenza B virus. Because influenza viruses exhibit ongoing antigenic changes, one or two of the vaccine viruses are updated almost every year.
The recommended timing for influenza vaccination is from September through mid-November. However, influenza activity frequently peaks after December and unvaccinated persons who are at high risk for complications should continue to be offered vaccine after November.
Live attenuated influenza virus (LAIV) vaccines have been under development since the 1960s. LAIVs have several potential advantages over inactivated influenza vaccine, including greater induction of mucosal IgA and intranasal administration as a spray or nose drops. Currently, studies on trivalent formulations of LAIV vaccines are under way.
The effectiveness of inactivated influenza vaccine depends in large part on the degree of match between circulating viral strains and vaccine strains as well as the age and health status of the recipient. In controlled trials among children and young adults, influenza vaccines are ~70-90% effective in reducing influenza when there is a good match between vaccine and circulating viruses. A meta-analysis of influenza vaccine studies among the elderly found the effectiveness of vaccines to be 56% in preventing illness, 50% in reducing hospitalization, and 68% for preventing death. In elderly nursing home residents, vaccine effectiveness is ~30% for preventing illness, but ~47-95% for reducing hospitalization, pneumonia, and death.
Each year, comprehensive recommendations on the use of influenza vaccines are published by the Centers for Disease Control and Prevention in an April or May issue of Morbidity and Mortality Weekly Report. In general, influenza vaccination is recommended for the groups.
Information on vaccinating people with human immunodeficiency virus (HIV) infection against influenza is limited. The main issues in question are whether persons with HIV are at elevated risk of influenza or serious complications from influenza, whether immunization poses a risk of accelerating HIV replication, and whether immunization is protective. Recent studies suggest that persons with HIV are at high-risk for developing complications from influenza. Because vaccine may result in protective antibody levels, it is felt that influenza vaccination will benefit many HIV-infected patients.
Side Effects of Vaccinations
The most common side effects of influenza virus vaccine consist of local effects, particularly soreness around the vaccination site, which occurs in as many as one-third of recipients. Fever, malaise, and myalgia are infrequent, and recent studies suggest that split-virus influenza vaccine does not lead to higher rates of systemic symptoms than placebo in young adults and the elderly.
Complications of Influenza Vaccination
The most serious potential neurologic complication of influenza vaccine is Guillain-Barre syndrome (GBS), a demyelinating condition of the peripheral nerves. The association between this disorder and influenza vaccine remains unclear, in large part because the disorder is uncommon both among persons who receive and do not receive influenza vaccine. The baseline annual incidence of GBS is about one to two cases per 100,000 adults. In 1976, administration of “swine flu” vaccine was associated with an increase of slightly under one case of GBS per 100,000 adults administered the vaccine. In addition, most of the risk for developing swine flu vaccine-associated GBS occurred within the 6 weeks after vaccine administration and peaked at 3 weeks after vaccination.
These vaccine-associated GBS cases were also less likely to be associated with antecedent illnesses and surgical procedures than other non-vaccine-associated GBS cases. Since then, four studies of influenza seasons between 1977 and 1991 did not find a statistically significant increase in the risk of GBS among vaccine recipients. However, a study of the 1992-93 and 1993–94 seasons found an increased risk of approximately one additional GBS case per million vaccinated persons. At this point, the association of GBS and influenza vaccines subsequent to the 1976 swine flu vaccine remains uncertain, but if these vaccines do pose a risk of GBS, the risk is quite small. Influenza vaccine is recommended for people at high risk for influenza-related complications because the risk of serious disease in these persons outweighs their risk of GBS.
Contraindications for Influenza Vaccination
Influenza vaccine is contraindicated in infants younger than 6 months because of the high risk of febrile reactions. Only split-virus preparations should be used in children = 13 years of age. Inactivated influenza vaccine is also contraindicated in people with acute febrile disease and persons known to have anaphylactic hypersensitivity reactions to eggs or other components of influenza vaccine. A physician should be consulted when a person at high risk for serious complications has such an allergic history; desensitization therapy and antiviral agents are options for preventing influenza in such persons.



