The herpesvirus group of the family Herpesviridae comprises large, enveloped, double-stranded DNA viruses found in both animals and humans. They are ubiquitous and produce infections ranging from painful skin ulcers to chickenpox to encephalitis. The major members of the group to infect humans are the two herpes simplex viruses (HSV-1 and -2), cytomegalovirus (CMV), varicella-zoster virus (VZV), Epstein-Barr virus (EBV), herpesvirus 6, and the recently discovered human herpesvirus types 7 and 8. Occasionally, the simian herpesvirus, herpes B virus, has caused human disease.
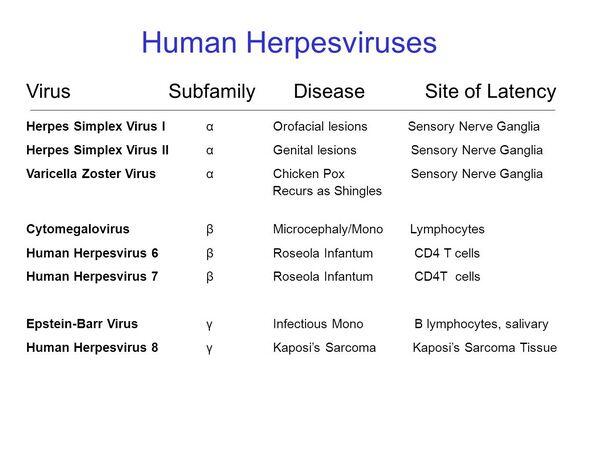
All herpesviruses are morphologically similar with an overall diameter of 180-200 nm. The nucleic acid core is ~ 30-45 nm in diameter, surrounded by an icosahedral capsid. The capsid is covered by a tegument and a lipoprotein envelope derived from the nuclear membrane of the infected host cell. The envelope contains at least nine glycoproteins that protrude beyond it as spikelike structures, while the tegument is a protein-filled area between the capsid and the envelope. Despite the morphologic similarity between these agents, substantial differences in the molecular composition of their genomes are reflected in their structural glycoproteins and polypeptides. Antigenic analysis is an important means for differentiation among herpesviruses despite some cross-reactions (eg, between HSV and VZV).
Susceptible tissue cultures vary significantly for the individual agents. HSV has the widest range; it replicates in numerous animal and human host cells. VZV is best grown in cells of human origin, although some laboratory-adapted strains can grow in primate cell lines. Human CMV replicates well only in human diploid fibroblast cell lines. EBV does not replicate in most commonly used cell culture systems but can be grown in continuous human or primate lymphoblastoid cell cultures. Human herpesvirus type 6 grows in lymphocyte cell cultures.
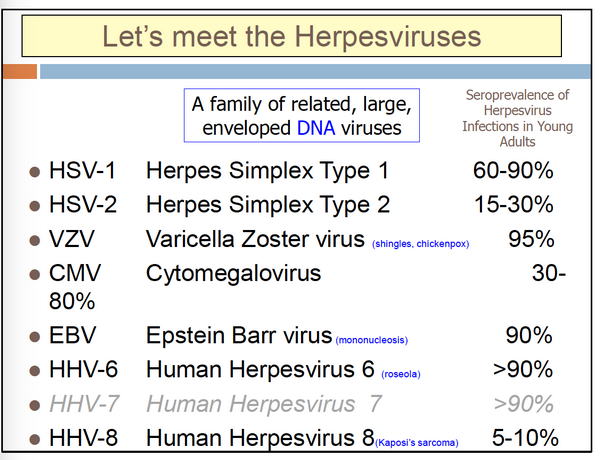
Characteristically, all of these agents produce an initial infection followed by a period of latent infection in which the genome of the virus is present in the cell, but infectious virus is not recovered. Reactivation of virus may then result in the first episode of clinically apparent disease or as recurrent disease. Complex host-virus interactions determine the expression of disease. With all of these agents, immunocompromised patients, especially those with altered cellular immunity, have more frequent and severe episodes, including clinically severe disease from reactivation of virus.
Herpes Simplex Virus
Varicella-Zoster Virus
Cytomegalovirus
Epstein-Barr Virus
Human Herpesvirus Type 6
OTHER HERPESVIRUSES
HERPESVIRUS TYPE 7
Isolation of human herpesvirus type 7 (HHV-7) was first reported in 1990. The virus was isolated from activated CD4+ T lymphocytes of a healthy individual. HHV-7 is distinct from all other known human herpesviruses but is most closely related to HHV-6. Seroepidemiologic studies indicate that this virus usually does not infect children until after infancy, and ~ 50% of infants are antibody positive by 2-4 years of age. As with HHV-6 this virus is frequently isolated from saliva, and close personal contact is the probable means of transmission. Also like HHV-6 this virus appears to be a cause of exanthem subitum. The diagnosis of acute infection can be made by the demonstration of seroconversion. No treatment has been identified.
HUMAN HERPESVIRUS TYPE 8 (Kaposi’s sarcoma-associated herpes virus [KSHV])
KSHV was discovered in 1994 by identification of unique viral DNA sequences in an AIDS patient’s Kaposi’s sarcoma (KS) tissue. The method used was representational difference analysis. Specific HHV-8 DNA sequences are found in the great majority of KS tissues in the United States, including those from non-AIDS cases, and occasionally in other specimens including lymphomas. Recently the virus was isolated in culture and, when characterized, seemed most closely related to EBV. Serologic and virologic data suggest that this virus is at least a cofactor in the pathogenesis of KS.
BOX 1. Herpes Simplex Infection
Children
Adults
More Common
- Mucocutaneous lesions, oral or genital
Less Common
- HSV stomatitis
- HSV encephalitis
- HSV disseminated
- HSV meningitis, encephalitis
BOX 2. Treatment of Herpes Simplex Infection
Children
Adults
First Choice
- Acyclovir
- Acyclovir
Second Choice
- Valacyclovir
- Famciclovir
BOX 3. Control of Herpes Simplex Infection
Prophylactic Measures
- Avoidance of contact with HSV positive lesions, secretions
- Daily acyclovir (OR valaciclovir, famiciclovir) suppresses recurrences of HSV infections
- Vaccine ineffective for treatment; under study for prophylaxis
BOX 4. Syndromes Caused by Varicella Infection
Children
Adults
More Common
- Varicella
- Zoster (shingles)
Less Common
- Zoster (shingles)
- VZ encephalitis
- Varicella
- VZ encephalitis
BOX 33-5. Treatment of Varicella Infection
Children
Adults
First Choice
- Acyclovir for children > 12 years who have varicella but no definite consensus
- Varicella: acyclovir, 800 mg 5 times/day
- Zoster: acyclovir, 800 mg 5 times/day; famciclovir, 500 mg/8 h; or valacyclovir, 1.0 g 3 times/day
Pediatric Considerations
- An antiviral agent is not recommended for children <12 years
BOX 6. Control of Varicella Infection
Prophylactic Measures
An attenuated VZV vaccine is now recommended for healthy children >1 year of age
VZ-specific immune globulin can diminish illness if given within 72 h of exposure. This is reserved for immunocompromised susceptible patients or other special circumstances
Isolation Precautions
If at all possible, patients with varicella or disseminated zoster should not be admitted to hospital; if unavoidable, these patients should be in strict isolation.
BOX 7. Cytomegalovirus Disease Syndromes
Children
Adults
More Common
Perinatal infection, “Daycare” infection
Less Common
Congenital infection
CMV retinitis (AIDS)
- pneumonia (transplant recipients)
- CMV enteritis, neurologic disease (all immunocompromised patients)
- CMV mononucleosis
BOX 8. Treament of Cytomegalovirus Disease
Children
Adults
First Choice
Ganciclovir
Ganciclovir, 5 mg/kg/d IV or valgauciclovir 900-1800 mg orally
Second Choice
- Foscarnet, 90-120 mg/kg/d IV
- Cidofovir, 5 mg/kg/q 2 weeks IV
Pediatric Considerations
No experience with treatments other than ganciclovir
BOX 9. Control of Cytomegalovirus Disease
Prophylactic Measures
- Avoid organ transplantation or blood donation from CMV seropositive to CMV seronegative
- Preemptive ganciclovir diminishes CMV disease in seropositive AIDS patients. Prophylactic ganciclovir or valacyclovir diminshes CMV disease in seropositive or mismatched transplant recipients. Preemptive ganciclovir disminshes CMV disease in seropositive or mismatched transplant recipients.
- Vaccine trials are in progress
BOX 10. Epstein-Barr Virus Infection
Children
Adults
More Common
Infectious mononucleosis
Less Common
EBV lymphoproliferaliferative disease
EBV lymphoproliferative disease
BOX 11. Treatment of Epstein-Barr Virus Infection
First Choice
None
Second Choice
Corticosteroids if severe toxicity or airway obstruction
Acyclovir and/or decrease immunosuppression for post transplant lymphoproliferative disease
BOX 12. Exanthem Subitum Syndromes
More Common
Exanthem subitum
Less Common
Meningitis, encephalitis fever in transplant recipients




