Essentials of Diagnosis
- Isolation of enterovirus in tissue culture.
- Detection of antigen or antibody not practical for routine diagnosis.
- Detection of enterovirus in cerebrospinal fluid (CSF) by polymerase chain reaction.
- CSF usually has lymphocytic pleocytosis with normal glucose and protein.
- Typical illness is febrile rash, macular or vesicular (hand, foot, mouth syndromes).
- Most clinical illness occurs in patients < 20 years old.
General Considerations
Enteroviruses are one of three types of picornaviruses that cause disease in humans. As the name indicates, picornaviruses are small (pico) ribonucleic acid (RNA) viruses that have a naked capsid structure. The family includes > 230 members divided into five genera but only three—enteroviruses, rhinoviruses, and hepatoviruses (hepatitis A virus)—cause human disease. These genera can be distinguished by their stability at pH 3, optimum temperature for growth, mode of transmission, and the diseases they cause. Rhinoviruses are discussed in site and the hepatitis A virus is discussed in site.
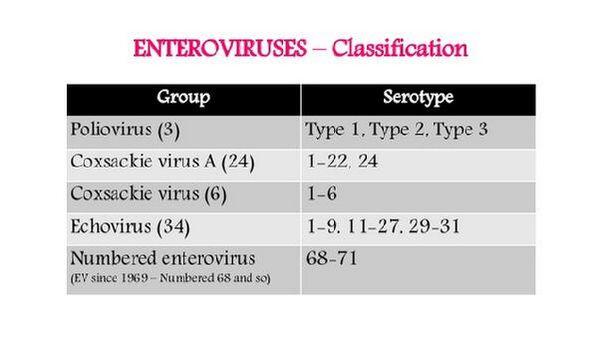
Nearly 70 serotypes of human enteroviruses exist, and they are divided into three subgroups: polio-, coxsackie-, and echoviruses. The capsids of these viruses are very stable in harsh environmental conditions (eg, sewage systems) and in the gastrointestinal tract, which facilitates their transmission by the fecal-oral route. Although they may initiate their infection in the gastrointestinal tract, the enteroviruses rarely cause enteric disease. Instead they cause myriad illnesses, especially diseases of the central nervous system and other systemic diseases. Several different disease syndromes may be caused by one enterovirus serotype, and several different serotypes may cause the same disease. The most well-known and well-studied picornavirus is poliovirus.
Coxsackieviruses are named after the town of Coxsackie, New York, where the viruses were first isolated. They are divided into two groups, A and B, on the basis of certain biologic and antigenic differences. These two groups are further subdivided into numeric serotypes by additional antigenic differences.
The name echovirus is derived from “Enteric Cytopathic Human Orphan,” because these agents were not thought to be associated with clinical disease. Thirty-one serotypes are now recognized. These viruses have a greater tendency than polioviruses to affect the meninges and cause meningitis, but a lesser tendency to infect anterior horn cells.
Epidemiology
The incubation period for enterovirus disease is usually 2-10 days but may be longer depending on the virus, the target tissue, and the age of the individual. Enteroviruses are highly contagious; poor sanitation and crowded living conditions foster transmission of enteroviruses, and sewage contamination of water supplies can result in enterovirus epidemics.
Humans are the major natural hosts of enteroviruses, and there is no evidence of spread from animals to humans. The enteroviruses are primarily spread person to person by enteric routes but may also be spread in droplets and cause respiratory infections. The most frequently isolated enteroviruses are coxsackieviruses A9, A16, and B1 to B5; and echoviruses 6, 9, 11, 16, and 30. These viruses are found worldwide, and each year there is a tendency for one of these to be the dominant circulating virus. Disease is most frequent in persons < 20 years of age but, as with poliovirus infection, it is generally less severe in children. However, coxsackie B virus and some of the echoviruses can cause severe disease. Secondary infections occur in = 70% of susceptible individuals living in households where the viruses have caused infection. Summer and fall are the major seasons for contracting enterovirus disease.
Microbiology
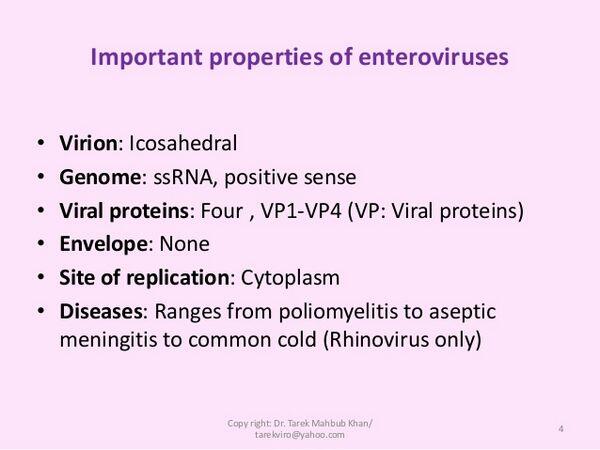
As with all picornavirus RNA, the RNA of the enteroviruses is surrounded by a very small icosahedral capsid ~ 30 nm in diameter. The genome of these viruses resembles messenger RNA (mRNA). It is a single strand of (+)-sense RNA of ~ 7.2-8.5 kilobases. It encodes a polyprotein that is proteolytically cleaved to produce the enzymatic and structural proteins of the virus. In addition to the capsid proteins, these viruses encode at least one protease and an RNA-dependent RNA polymerase.
The specificity of enterovirus interaction with cellular receptors is the major determinant of their tissue tropism and the diseases they cause. The VP1 proteins at the vertices of the virion contain a canyon into which the receptor binds. The receptors for polioviruses have recently been identified as tissue-specific intercellular adhesion molecules (ICAMs), which are members of the immunoglobulin super- family. Several serotypes of coxsackievirus recognize intercellular adhesion molecule 1 (ICAM-1). ICAM-1 is expressed on epithelial cells, fibroblasts, and endothelial cells. Poliovirus binds to a molecule of similar structure. The cells in which the poliovirus receptor is expressed correlate directly with the organs infected by poliovirus infection.
On binding to the receptor, the enteroviruses are internalized by receptor-mediated endocytosis, and the virions dissociate in the acidic environment of the endosome, releasing the genome into the cytoplasm. The genome then binds to ribosomes, and a polyprotein is synthesized within 10-15 min of infection. The polyprotein is initially cleaved by cellular proteases until a viral protease is generated to cleave the rest of the polyprotein.
The RNA-dependent RNA polymerase generates a negative-strand RNA template from which the new mRNA genome and templates can be synthesized. The amount of viral mRNA increases rapidly in the cell, with the number of viral RNA molecules reaching 400,000/cell.
Cellular RNA and protein synthesis are inhibited during infection by several enteroviruses; a viral protease blocks translation of cellular mRNA, and permeability changes induced by enteroviruses reduce the ability of cellular mRNA to bind to the ribosome. Viral mRNA also competes with cellular mRNA for the factors required in protein synthesis. These activities contribute to the cytopathic effect of the virus on the target cell.
As the viral genome is being replicated and translated, the structural proteins are cleaved from the polyproteins. After insertion of the viral genome, assembly of viral RNA into the viral capsid occurs in the cytoplasm, and the virion is released when lysis and destruction of the cell occur.
Pathogenesis
Differences in pathogenesis of the enteroviruses mainly result from differences in tissue tropism of the various subgroups. Poliovirus has been studied extensively and is the prototype for the pathogenesis of the enteroviruses.
The upper respiratory tract, the oropharynx, and the intestinal tract are the portals of entry for the enteroviruses. The virus initiates replication in the mucosa and lymphoid tissue of the tonsils and pharynx and later infects the gut. The virions are impervious to stomach acid, proteases, and bile and infect lymphoid cells of Peyer’s patches and the intestinal mucosa. Primary viremia spreads the virus to receptor-bearing target tissues, where a second phase of viral replication may occur, resulting in symptoms and a secondary viremia. Virus shedding from the oropharynx can be detected for a short time before symptoms begin, whereas virus production and shedding into stool may last for = 4 months, even in the presence of a humoral immune response.
The nature of the enterovirus disease is determined by the tissue tropism of the virus. Poliovirus has one of the narrowest tissue tropisms, recognizing a receptor expressed on anterior horn cells of the spinal cord, dorsal root ganglia, motor neurons, and few other cells. Coxsackieviruses and echoviruses recognize receptors expressed on more cell types and tissues and cause a broader repertoire of diseases. Coxsackievirus and echovirus receptors may be found in the central nervous system and on heart, lung, pancreatic, and other cells. Differences in the susceptibility to and severity of poliovirus and coxsackievirus infection with age may also be attributed to differences in distribution and amount of receptor expression. Adults are generally more susceptible to serious disease with poliovirus, whereas newborns experience the most serious symptoms from coxsackie B and echovirus infections.
Most enteroviruses are cytolytic, replicating rapidly and causing direct damage to the target cell. Histologic examination reveals cell necrosis and mononuclear cell infiltrates.
The production of antibody is the major protective immune response to enteroviruses. Secretory antibody can prevent the initial establishment of infection in the oropharynx and gut, and serum antibody prevents viremic spread to the target tissue. However, in the individual with poor immune response, antibody production may be too late to block infection of the target tissue, and patients with a deficiency of antibody production may have persistent enterovirus infections. Serum-neutralizing antibody generally develops 7-10 days after the initial onset of infection.
Cell-mediated immunity is not likely to be involved in protection but may play a role in pathogenesis. T cells appear to contribute to coxsackie B virus-induced myocarditis in mice. Indeed, certain late enterovirus syndromes (eg, myocarditis, myositis, and nephritis) may be immunologically mediated rather than resulting from direct viral invasion. Immune responses to the virus may cross-react with cellular antigens.
Enteroviruses: Clinical Syndromes
Box 1. Enterovirus Infection
Pediatric
Adult
More Common
- Febrile rash
- Meningitis
- Meningitis
Less Common
- Hand-foot-mouth
- Herpangina syndrome
- Neonatal “sepsis”
- Pleurodynia
- Myopericarditis
BOX 2. Treatment of Enterovirus Infection
First Choice
Immune globulin for chronic infection of CNS in incubation period
Pediatric Considerations
See above
BOX 3. Control of Enterovirus Infection
Prophylactic Measures
- Poliovirus vaccine, which induces a protective antibody response
- No vaccines exist for coxsackieviruses or echoviruses. Transmission can presumably be reduced by improvements in hygiene and living conditions
Isolation Precautions
- Careful handwashing after patient contact
- Stool precautions

