Definitions
- Immunization is the process of introducing an antigen into the body to induce protection against an infectious agent without causing disease.
- Vaccines are substances administered to generate a protective immune response.
- Toxoids are inactivated bacterial toxins. They retain the ability to stimulate the formation of antitoxin.
- Adjuvants are inert substances, such as aluminum salts (i.e., alum), which enhance vaccine antigenicity by prolonging antigen absorption.
- Immune sera are sterile solutions containing antibody derived from human (immune globulin) or equine (antitoxin) sources.
- Antitoxins contain antibodies that are made by immunizing animals with an antigen and then harvesting the antibodies from serum.
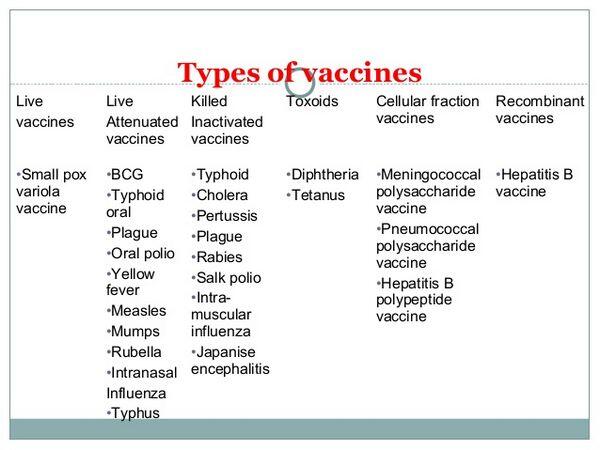
Vaccine and toxoid recommendations
- In general, inactivated vaccines can be administered simultaneously at separate sites. Killed and live antigens may be administered simultaneously or, if they cannot be administered simultaneously, at any interval between doses with the exception of cholera (killed) and yellow fever (live) vaccines, which should be given at least 3 weeks apart. If live vaccines are not administered simultaneously, their administration should be separated by at least 4 weeks.
- Vaccination of pregnant women generally is deferred until after delivery because of concern over potential risk to the fetus. Administration of live attenuated vaccines should not be done during pregnancy, and inactivated vaccines may be administered to pregnant women when the benefits outweigh the risks. Hepatitis A, hepatitis B, meningococcal, inactivated polio, and pneumococcal polysaccharide vaccines should be administered to pregnant women who are at risk for contracting these infections.
- Patients with chronic conditions that cause limited immune deficiency (e.g., renal disease, diabetes, liver disease, and asplenia) and who are not receiving im- munosuppressants may receive live attenuated and killed vaccines, and toxoids.
- Patients with active malignant disease may receive killed vaccines or toxoids but should not be given live vaccines. Live virus vaccines may be administered to persons with leukemia who have not received chemotherapy for at least 3months.
- If a person has been receiving high-dose corticosteroids or have had a course lasting longer than 2 weeks, then at least 1 month should pass before immunization with live virus vaccines.
- Responses to live and killed vaccines generally are suboptimal for HIV-infected patients and decrease as the disease progresses.
- General contraindications to vaccine administration include a history of anaphylactic reaction to a previous dose or an unexplained encephalopathy occurring within 7 days of a dose of pertussis vaccine. Immunosuppression and pregnancy are temporary contraindications to live vaccines.
Diphtheria toxoid adsorbed (DTA) and Diphtheria antitoxin (DA)
- Two strengths of diphtheria toxoid are available (pediatric [D] and adult [d], which contains less antigen). Primary immunization with DTA is indicated for children less than 6 weeks of age. Generally, DTA is given along with acellular pertussis and tetanus vaccines (DTaP) at 2, 4, and 6 months of age, and then at 15 to 18 months and 4 to 6 years of age.
- For nonimmunized adults, a complete three-dose series of diphtheria toxoid should be administered, with the first two doses given at least 4 weeks apart and the third dose given 6 to 12 months after the second. The combined preparation, Td, is recommended in adults because it contains less diphtheria toxoid than DTaP, with fewer reactions seen from the diphtheria preparation. Booster doses are given every 10 years.
- DA is a sterile diphtheria antitoxin derived from hyperimmunized horses and is indicated for immediate use in patients with diphtheria. Sensitivity testing by performing an intradermal or scratch test and a conjunctival test should be performed before administration.
- The usual dose of DA is 20,000 to 40,000 units for pharyngeal disease, 40,000 to 60,000 units for nasopharyngeal lesions, and 80,000 to 120,000 units for extensive disease of 3 or more days.
Tetanus toxoid, tetanus toxoid adsorbed, and tetanus immune globulin
Haemophilus influenzae type (HIB) vaccines
- Hib vaccines currently in use are conjugate products, consisting of either a polysaccharide or oligosaccharide of polyribosylribitol phosphate (polyribosyl-ribitol-phosphate) covalently linked to a protein carrier.
- Hib conjugate vaccines are indicated for routine use in all infants and children less than 5 years of age.
- The primary series of Hib vaccination consists of 0.5-mL intramuscularly doses at 2, 4, and 6 months of age (for HibTITER [HbOC] and ActHIB [polyribosyl-ribitol-phosphate-T]) or doses at 2 and 4 months if polyribosyl-ribitol-phosphate-OMP is used (Table 49-4). A booster dose is recommended at age 12 to 15 months.
- For infants aged 7 to 11 months who have not been vaccinated, three doses of HbOC, polyribosyl-ribitol-phosphate-OMP, and polyribosyl-ribitol-phosphate-T should be given: two doses, spaced 4 weeks apart, and then a booster dose at age 12 to 15 months (but at least 8 weeks since dose 2). For unvaccinated children aged 12 to 14 months, two doses should be given, with an interval of 2 months between them. In a child older than 15 months, a single dose of any of the four conjugate vaccines is indicated.
Influenza virus vaccine
- Annual influenza vaccination is strongly recommended for individuals over the age of 6 months with chronic medical conditions that make them at increased risk for the complications of influenza. Indications for annual influenza vaccination are as follows:
- All individuals 50 years of age and older
- Residents of nursing homes
- Adults and children with chronic cardiovascular or pulmonary diseases including asthma
- Adults and children with chronic metabolic disease, renal dysfunction, hemoglobinopathies, or immunosuppression (including immunosuppression from drugs or HIV)
- Children and teenagers receiving chronic aspirin therapy
- Pregnant women
- Health care workers
- Employees of residential care facilities for high-risk patients
- Household members of persons in high-risk groups.
- Individuals who should not be vaccinated are those with anaphylactic hypersensitivity to eggs or other components of the vaccine or adults with febrile illness (until the fever abates).
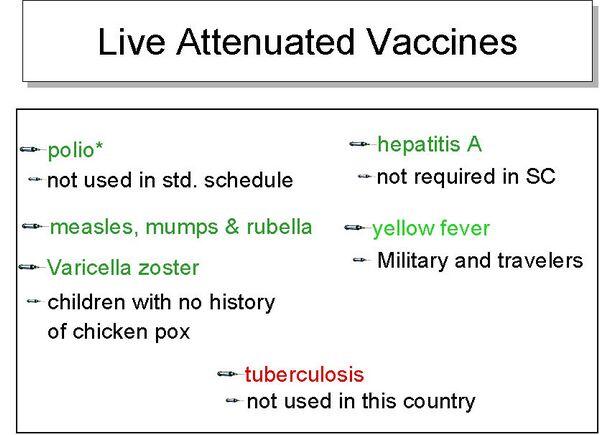
Measles vaccine
- Measles vaccine is a live attenuated vaccine that is administered for primary immunization to persons 12 to 15 months of age or older, usually as a combination of measles, mumps, and rubella. A second dose is recommended at 4 to 6 years of age.
- The vaccine should not be given to immunosuppressed patients (except those infected with HIV) or pregnant women.
- The vaccine should not be given within 1 month of any other live vaccine unless the vaccine is given on the same day (as with the measles, mumps, and rubella vaccine).
- Measles vaccine is indicated in all persons born after 1956 or in those who lack documentation of wild virus infection either by history or antibody titers.
- For postexposure prophylaxis, the vaccine is effective if given within 72 hours of exposure. In addition, immune globulin may be administered intramuscularly at a dose of 0.25 mg/kg (maximum dose 15 mL), if given within 6 days of exposure.
Meningococcal polysaccharide vaccine
Meningococcal polysaccharide vaccine is indicated in high-risk populations such as those exposed to the disease, those in the midst of uncontrolled outbreaks, travelers to an area with epidemic hyperendemic meningococcal disease, or individuals who have terminal complement deficiencies or asplenia.
- The vaccine should be made available to students starting college who wish to decrease their risk for meningococcal disease.
- The vaccine is administered subcutaneously as a single 0.5-mL dose.
Mumps vaccine
- The vaccine (usually given in conjunction with measles and rubella, measles, mumps, and rubella) is given beginning at age 12 to 15 months, with a second dose prior to entry into elementary school. If the vaccine is given before 12 months of age, revaccination is necessary and should be given after reaching 1 year of age.
- The vaccine is also indicated in previously unvaccinated adults and in those in whom a poor history of wild virus infection or previous administration of killed mumps exists.
- Postexposure vaccination is of no benefit.
- Mumps vaccine should not be given to pregnant women or immunosuppressed patients. The vaccine should not be given within 6 weeks (preferably 3 months) of administration of immune globulin.
Pertussis vaccine
- Acellular pertussis vaccine is usually administered in combination with diphtheria and tetanus toxoids (as DTaP).
- The primary immunization series for pertussis vaccine consists of four doses given at ages 2, 4, 6, and 15 to 18 months. A booster dose is recommended at age 4 to 6 years.
- Systemic reactions, such as moderate fever, occur in 3% to 5% of those receiving vaccines. Very rarely, high fever, febrile seizures, persistent crying spells, and hypotonic hyporesponsive episodes occur after vaccination.
- There are only two absolute contraindications to pertussis administration: an immediate anaphylactic reaction to a previous dose or encephalopathy within 7 days of a previous dose, with no evidence of other cause.
Pneumococcal vaccine
Poliovirus vaccines
- Two types of trivalent poliovirus vaccines are currently licensed for distribution in the United States: an enhanced inactivated vaccine and a live attenuated, oral vaccine. inactivated vaccine is the recommended vaccine for the primary series and booster dose for children in the United States, whereas oral vaccine is recommended in areas of the world that have circulating poliovirus.
- inactivated vaccine is given to children aged 2, 4, and 6 to 18 months and 4 to 6 years. Primary poliomyelitis immunization is recommended for all children and young adults up to age 18 years. Allergies to any component of inactivated vaccine, including streptomycin, polymixin B, and neomycin, are contraindications to vaccine use.
- Oral vaccine is not recommended for persons who are immunodeficient or for normal individuals who reside in a household where another person is immunodeficient. Oral vaccine should not be given during pregnancy because of the small but theoretical risk to the fetus.
Rubella vaccine
- The vaccine is given with measles and mumps vaccines at 12 to 15 months of age, then at 4 to 6 years.
- The vaccine should not be given to immunosuppressed individuals, although measles, mumps, and rubella vaccine should be administered to young children with HIV without severe immunosuppression as soon as possible after their first birthday. The vaccine should not be given to individuals with anaphylactic reaction to neomycin.
- Although the vaccine has not been associated with congenital rubella syndrome, its use in pregnancy is contraindicated. Women should be counseled not to become pregnant for 4 weeks following vaccination.
Varicella vaccine
- Varicella virus vaccine is recommended for all children 12 to 18 months of age and for persons above this age if they have not had chickenpox. Persons aged 13 years and older should receive two doses separated by 4 to 8 weeks.
- The vaccine is contraindicated in immunosuppressed or pregnant patients.
- Children with asymptomatic or mildly symptomatic HIV should receive two doses of varicella vaccine 3 months apart.
Varicella-zoster immune globulin
- Varicella-zoster immune globulin is used for passive immunization of susceptible immunodeficient patients exposed to varicella-zoster infection.
- Use of Varicella zoster immune globulin should be considered in exposed children and certain adults who are immunocompromised and susceptible to varicella-zoster. Conditions warranting consideration of Varicella zoster immune globulin after varicella-zoster virus exposure are as follows:
- Children with primary or acquired immunodeficiency, neoplastic disease, or who require immunosuppressive therapy
- Neonates whose mothers develop varicella within 5 days before or 2 days after delivery
- Preterm infants (less than 28 weeks’ gestation or who weigh less than 1000 g) who are exposed to varicella while hospitalized
- Susceptible pregnant women
- Immunosuppressed adults and adolescents
- For maximum effectiveness, Varicella zoster immune globulin must be given within 48 hours and not more than 96 hours following exposure.
- Administration of Varicella zoster immune globulin is by the intramuscular route (never intravenously).
Immune globulin
- Immune globulin is available as both intramuscular (IGIM) and intravenous (IGIV) preparations.
| TABLE. Indications and Dosage of Intramuscular Immune Globulin in Infectious Diseases | ||||||||||||||
|
- Table Indications and Dosage of Intramuscular Immune Globulin in Infectious Diseases lists the suggested dosages for IGIM in various disease states.
- The uses for IVIG are as follows:
- Primary immunodeficiency states including both antibody deficiencies and combined deficiencies
- Idiopathic thrombocytopenic purpura
- Chronic lymphocytic leukemia in patients who have had a serious bacterial infection
- Kawasaki disease (mucocutaneous lymph node syndrome)
- Bone marrow transplant
- Varicella-zoster
Rho(D) Immune globulin (RDIg)
- RDIg suppresses the antibody response and formation of anti-Rho(D) in Rho(D)-negative, Du-negative women exposed to Rho(D)-positive blood and prevents the future chance of erythroblastosis fetalis in subsequent pregnancies with a Rho(D)-positive fetus.
- RDIg, when administered within 72 hours of delivery of a full-term infant, reduces active antibody formation from 12% to between 1 and 2%.
- RDIg is also used in the case of a premenopausal woman who is Rho(D) negative and has inadvertently received Rho(D)-positive blood or blood products.
- RDIg may be used after abortion, miscarriage, amniocentesis, or abdominal trauma.
- RDIg is administered intramuscularly only.


 (1 votes, average: 4.00 out of 5)
(1 votes, average: 4.00 out of 5)