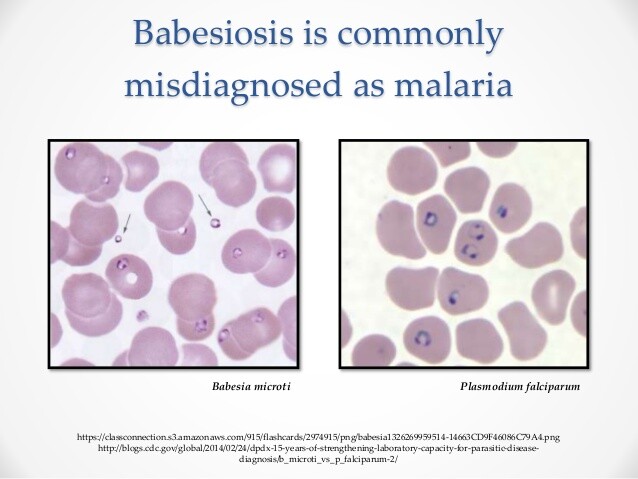
PLASMODIUM SPP.
- Exposure history, such as travel, recent transfusion, or living in close proximity to an international airport.
- Nonfalciparum malaria: chills and fever spikes, followed by defervescence and fatigue; symptoms may be cyclic every 48-72 h.
- Falciparum malaria: fever spikes and chills, often noncyclic and associated with rapidly progressive systemic symptoms.
- Detection and identification of a Plasmodium species in a thick and thin blood smear, respectively.
- Molecular detection of P falciparum’s histidine-rich protein by enzyme-linked immunosorbent assay (ELISA) or Plasmodium DNA by polymerase chain reaction (PCR) followed speciation by probe hybridization or DNA sequencing.
General Considerations
Epidemiology
Malaria, a disease of antiquity, was recognized by Hippocrates and described possibly as early as 1700 BC in ancient Chinese texts. Malaria is a global disease that occurs most commonly in the tropics; however, transmission may also occur in temperate zones. In the 19th and early 20th centuries, Plasmodium species were widely distributed in the United States. This distribution included the southern United States, the Mississippi River Valley, and extensions as far north as Minnesota and Michigan.
Today, primarily in tropical areas, Plasmodium parasites continue to cause > 100 million cases of malaria per year. This results in an estimated 1-2 million deaths annually, many of whom are children. In fact, greater than 90% of severe, life-threatening malaria occurs in children. The distribution of the mosquito vector and the prevalence of disease in indigenous populations are the major factors that determine the distribution of the Plasmodium parasite.
Mosquito-infested areas, such as swamps, have long been associated with high attack rates of malaria. Environments that support long-standing, stagnant water promote mosquito breeding. Currently, endemic areas include parts of the Caribbean, northern South America, Central America, parts of Africa, India, parts of Australia, Southeast Asia, and many of the Asian Pacific Islands.
Malaria also occurs sporadically in nonendemic areas. In many instances, this represents imported, latent disease. Malarial relapses may present months after travelers have returned from endemic areas. These patients have usually been incompletely treated or have taken insufficient chemoprophylaxis.
Relapsing malaria is caused by reactivation of the latent hypnozoite phase of P vivax or P ovale. Patients who develop malaria may be treated with a wide variety of agents. The most commonly used antimalarial drugs, chloroquine and mefloquine, are effective against the symptomatic, erythrocytic phase of P vivax and P ovale and may result in apparent cures. These drugs, however, are ineffective against the hepatic hypnozoites. Patients treated in such a manner are incompletely treated and are at risk for malarial relapse.
Some patients may report never having had a previous episode of malaria. Specific questioning, however, often reveals a brief lapse in chemoprophylaxis. A lapse in chemoprophylaxis may result in a window period of subprophylactic drug levels. During this window period, sporozoites injected by an infected mosquito during a blood meal may reach and infect the liver. Any parasites (merozoites) that emerge from the liver while the patient is taking chemoprophylaxis are rapidly killed and the patient remains asymptomatic. Hypnozoites, however, may become active months after return from an endemic area, long after the cessation of chemoprophylaxis. The proper identification of P vivax and P ovale is important because only these species form hepatic hypnozoites and may result in malarial relapse.
In nonendemic areas, the cases of so-called “airport malaria” may occur. It is thought that mosquito vectors from endemic areas may be transported with airline cargo. An individual in a nonendemic area who lives in close proximity to an airport may become infected if bitten by these mosquitos. The propagation of the parasite is usually not sustained in the environment. This is either because of a lack of a suitable mosquito host or because of the low number of parasites in the community. When the prevalence of malaria is low, the probability of a mosquito ingesting gametocytes is very low. Additionally, if infected mosquitos are rare, they may take a nonhuman blood meal and thereby disrupt the parasitic cycle.
Mosquito transmission is the most common route of infection, but other modes of transmission exist. Transmission may result from intravenous drug abuse (shared needles) or blood transfusion. In these instances, only the erythrocytic cycle is established, because hepatocytes can be infected only by the sporozoite form of the parasite.
Microbiology
Numerous Plasmodium species exist; however, only four species are known to infect humans. These species are P falciparum, P vivax, P ovale, and P malariae. Worldwide, P vivax causes the vast majority of disease (~ 80%), followed by P falciparum (~ 15%).
Depending on the infecting plasmodial organism, malaria differs in severity, complications, prognosis, and treatment. Therefore, with the exception of differentiating P vivax from P ovale, it is important to promptly and properly speciate the organism. Malaria is typically separated into two disease types: falciparum and nonfalciparum. This distinction emphasizes the severity of P falciparum malaria, which is a medical emergency in the nonimmune individual. Among the nonfalciparum species, it is important to distinguish P vivax and P ovale from P malariae. The former two species have a dormant hepatic stage, which requires separate treatment to avoid malarial relapse.
The identification of Plasmodium organisms and the differentiation of the various species remain primarily based on parasite morphology in Giemsa-stained blood preparations. Organism detection is the initial task in the laboratory diagnosis of malaria. This is accomplished by the examination of a thick blood preparation. The examination of a thick preparation is an important part of the examination of blood for parasites and should not be bypassed, unless plasmodia have already been detected in a routine peripheral blood smear. The thick preparation increases the diagnostic yield compared with the examination of the thin blood smears. In the thick preparation, a larger volume of blood may be screened more rapidly. This becomes critical in detecting low-grade parasitemia, such as that associated with long-term P malariae infections or early in the course of malaria.
Thick preparations are made by placing 1-2 drops of the patient’s blood together on a glass slide. The blood is not smeared and allowed to air dry. This slide is then stained by the Giemsa method, without methanol fixation. The unfixed erythrocytes (RBCs) lyse in the hypotonic stain solution. The stained preparation is first examined under 10× magnification, then under 100× oil immersion.
Filariasis is endemic to many of the same regions that harbor the malaria parasites and may cause cyclic fevers. The microfilariae are easily detected in thick blood preparations at 10× magnification. The purpose of the 100× oil immersion examination is to detect Plasmodium species.
In Giemsa-stained preparations, the Plasmodium parasites have red chromatin and light-blue cytoplasm. The ring forms of any Plasmodium species and the gametocytes of P falciparum are the structures most easily identified in thick preparations. Practice is required to differentiate Plasmodium amoeboid and schizont forms from platelets and debris. Thin blood smears must be examined if the thick preparations demonstrate the presence of a Plasmodium species.
The methanol-fixed, Giemsa-stained thin blood smear is used for Plasmodium speciation. Useful criteria for the differentiation of Plasmodium species are listed in Table 80-1. However, only rarely are all of these morphologic features present in a blood smear. Differentiation must be made on all information available. The morphologic criteria useful in differentiating the Plasmodium species are briefly discussed.
The crescent-shaped gametocyte is pathognomonic of P falciparum, but, unfortunately, this structure is not always present. Features in the peripheral smear, that in combination may be used to identify P falciparum, include the presence of small, delicate-appearing ring trophozoites, infected erythrocytes that remain normocytic, and the presence of predominantly ring trophozoites, with a conspicuous absence or relative rarity of more advanced forms. A high parasitemia and erythrocytes infected with multiple organisms are more commonly seen in patients with falciparum malaria. Applique forms and two chromatin centers per ring trophozoite are also suggestive of P falciparum, but may be present in other Plasmodium species.
Unlike P falciparum, P vivax and P ovale produce thicker and larger ring trophozoites, the infected RBCs become macrocytic, and advanced forms, such as amoeboid trophozoites, schizonts, or both are usually present in the peripheral smear. In an appropriately pH-balanced Giemsa stain (pH 6.8-7.0), RBCs infected by P vivax or P ovale may demonstrate fine eosinophilic stippling. This fine stippling should not be confused with larger, coarse, comma-shaped dots (Maurer’s dots) that may be present in P falciparum-infected erythrocytes. Applique forms are typically not present in P vivax- or P ovale-infected erythrocytes, and ring trophozoites usually have only a single chromatin dot. Occasionally, more than one trophozoite may be present per erythrocyte. Although P vivax and P ovale can be differentiated from one another, this is difficult and requires parasitology expertise. Differentiation of P vivax and P ovale is usually not indicated or performed, because the disease produced by these organisms is similar and the treatment is identical.
P malariae-infected RBCs invariably contain some advanced plasmodial forms and, like P vivax and P ovale, the ring forms produced are thick. Stippling, however, is not observed. Specialized advanced forms, such as the band form and the basket form, are highly suggestive of P malariae. Like P falciparum, the ring trophozoites may occasionally contain two chromatin dots and the infected RBCs remain normocytic.
It should be noted that there may be coinfections with more than one Plasmodium species. Coinfections, especially in individuals who live in areas endemic for more than one Plasmodium species, are relatively common. These mixed infections are important to detect because of differences in disease prognosis and therapy.
Pathogenesis
The definitive host and vector for the Plasmodium parasite is the female Anopheles mosquito. Asexual reproduction and gametogenesis occur in the human intermediate host. The parasitic life cycle is similar for all Plasmodium species. However, important differences do exist.
Infecting sporozoites originate from the salivary gland of the female Anopheles mosquito. These are transmitted into the human during a blood meal. The sporozoites then migrate via the bloodstream to the liver where hepatocytes become infected. In the liver, tissue schizonts are formed. These contain numerous, asexually derived merozoites. From a single sporozoite, this phase of asexual reproduction results in a 10,000- to 30,000-fold organism amplification. This portion of the asexual reproduction cycle is common to all Plasmodium species.
Unique to the life cycle of P vivax and P ovale is the production of dormant hepatic hypnozoites. The hepatic hypnozoites may represent a form of parasite adaptation to climate. In temperate zones, this would enable the malarial parasite to “over-winter” in the human host. This strategy would prove advantageous when climatic conditions limit the activity of the mosquito vector. The hypnozoites, after a dormancy period between 6 and 12 months, become active and produce tissue schizonts.
Upon maturation, the tissue schizonts rupture, and the merozoites are released into the bloodstream. The merozoites then infect RBCs wherein occurs the second phase of asexual reproduction. Intra-erythrocytic asexual replication is also common to all Plasmodium species. P falciparum and P malariae may invade erythrocytes of any age, whereas P vivax and P ovale selectively parasitize only young RBCs. Younger RBCs still maintain their full complement of cytoplasmic membrane and expand with the growth of the organism. Older RBCs, infected with either P falciparum or P malariae, fail to expand with the growth of the parasite and remain normocytic with respect to uninfected RBCs. These features are useful in the laboratory differentiation of Plasmodium species.
After RBC infection, there is another cycle of asexual replication. Initially, a ring form develops, followed by differentiation into an amoeboid trophozoite form. This is followed by the development of an intra-erythrocytic schizont, which contains many merozoites. This phase results in a 6- to 32-fold asexual amplification of the organism for each infected RBC. The number of merozoites produced in the intra-erythrocytic schizont varies between species. Schizonts present in the blood smear are useful for speciation. The developing parasites metabolize glucose and use RBC hemoglobin. The parasitic use of hemoglobin produces the characteristic hemozoin pigment as a waste product.
The erythrocyte-based, asexual reproduction cycle culminates with RBC rupture. The merozoites are released into the bloodstream where erythrocytes are again infected. This cycle of erythrocyte infection, merozoite replication, and RBC rupture is repetitive and may become highly synchronized. This synchronization is most classically seen in benign tertian malaria caused by P vivax. The rupture of the RBCs and release of the merozoites correlate with the clinical symptoms of malaria. The RBC-based reproduction cycle of P vivax and P ovale occurs every 48 h, whereas the erythrocytic cycle of P falciparum occurs between 36 and 48 h. The erythrocytic cycle of P malariae occurs approximately every 72 h.
The RBC-infecting merozoite may alternatively undergo differentiation into either a micro- or macrogametocyte. These gametocytes may be ingested by the female Anopheles mosquito during a blood meal. Fusion of the gametocytes takes place within the gut of the mosquito. The diploid zygote matures and invades the gut wall. Meiotic division ensues, which results in haploid sporozoites. The sporozoites migrate to the mosquito’s salivary gland to complete the parasitic cycle.
Nonfalciparum Malaria (P Vivax, P Ovale, P Malariae)
Table 1. Useful differentiating characteristics of Plasmodium species.a
Morphologic Feature
P falciparum
P malariae
P vivax/P ovale
RBCs
- Infected RBC size
- RBC stippling
- Normocellular
- Coarse dots, commalike, occasionally present (Maurer’s dots)
- Normocellular
- None present
- Increased
- Fine stippling (Schuffner’s dots — P vivax); (Jame’s stippling — P ovale)
Rings
- Parasite load
- > 1 organism/cell
- Applique forms
- Often high
- Common
- Present
- Often low
- Low
- Usually absent
- Intermediate
- Occasional
- Usually absent
Advanced forms
- Advanced forms
- Ameboid forms
- Number of merozoites/cell
- Amount of RBC occupied by schizont
- Distinctive “banana-shaped” gametocyte
- Usually absent, except in severe disease
- N/A
- N/A
- N/A
- Present
- Present
- Basket, band, and indistinct amoeboid forms
- 6-12 (average 8)
- Entire cell
- Absent
- Present
- Indistinct forms
- P vivax: 12-24 (average 16)
- P ovale: 8-12 (average 8)
- P vivax: entire cell
- P ovale: fills 2/3 of cell
- Absent
Other Features
- Cycle of fever
- Hypnozoitesb
- Usually without established cycle
- Absent
- 72 h
- Absent
- 48 h
- Present
- Knowledge of species endemic to the area wherein the patient contracted malaria is also useful in limiting the diagnostic possibilities.
- In nonendemic areas, this is often demonstrated by reactivation several months after leaving an endemic area.
BOX 1. Plasmodium-Associated Syndromes
P vivax/
P ovale
Cyclic episodes that consist of chills followed by fever, which is followed by defervescence and diaphoresis; cyclic every 48 h
P malariae
Cyclic episodes that consist of chills, followed by fever, which is followed by defervescence and di-aphoresis; cyclic every 72 h; possible immune-complex — mediated glomerulonephritis
P falciparum
Continuous fevers with irregular spikes, possible hyperparasitemia with microvascular damage and compromise. This is a medical emergency in the nonimmune. Microvascular compromise may lead to central nervous system damage, renal and pulmonary failure, and death.
BOX 2. Treatment of Malaria
Children
Adults
P falciparum malaria from areas with chloroquine resistance1,2
First Choice
- Mefloquine, 25 mg/kg, not to exceed the adult dosage, PO, taken with = 8 oz of water (single dose)
- Mefloquine, five 250-mg tablets (1250 mg) PO, with = 8 oz of water (single dose)
Second Choice
Quinine sulfate, 25 mg/kg/d PO with one of the following:
- Doxycycline,3 2 mg/kg/d for 7 d, given with the quinine
OR
- Followed by pyrimethamine-sulfadoxine (0.25 tablet: age <1 yr; 0.5 tablet, age 1-3 yr; 1.0 tablet, age 4-8 yr; 2 tablets, age 9-14 yr
OR
- Followed by clindamycin, 20-40 mg/kg/d in 3 divided doses
- Quinine sulfate, 650 mg PO every 8 h for 3-7 d, with one of the following:
- Doxycycline, 100 mg twice daily for 7 d
OR
- Followed by 3 pyrimethamine-sulfadoxine tablets.
OR
- Followed by clindamycin, 900 mg three times a day for 5 d
Parenteral Therapy
- Quinine dihydrochloride, 20 mg of salt/kg IV loading dose in 5% dextrose given over 4 h, followed by 10 mg of salt/kg given over 2-4 h, every 8 h (maximum dose, 1800 mg/d)
OR
- Quinidine gluconate, 10 mg salt/kg loading dose in normal saline, with slow infusion lasting 1-2 h, (maximum dose, 600 mg), followed by continuous infusion at 0.02 mg/kg/min
- Quinine dihydrochloride, 20 mg of salt/kg IV loading dose in 5% dextrose given over 4 h, followed by 10 mg of salt/kg given over 2-4 h, every 8 h (maximum dose, 1800 mg/d)
OR
- Quinidine gluconate, 10 mg salt/kg loading dose in normal saline, with slow infusion lasting 1-2 h, (maximum dose, 600 mg), followed by continuous infusion at 0.02 mg/kg/min
Malaria caused by P vivax, P ovale, P malariae, and chloroquine-sensitive
P falciparum
First Choice
- Chloroquine, 10 mg base/kg PO loading dose (not to exceed 600 mg base), followed by 5 mg base/kg (not to exceed 300 mg base) given 6 h after the first dose and again on days 2 and 3
- Chloroquine, 600 mg base (1000 mg chloroquine phosphate) PO loading dose, followed by 300 mg base given 6 h after the first dose and again on days 2 and 3
Latent disease caused by P vivax/P ovale
First Choice
- Primaquine phosphate, 0.3 mg base (0.5 mg salt) /kg/d × 14 d
- Primaquine phosphate, 15.3 mg base (26.5 mg salt) qd PO × 14 d
OR
- 45 mg base (79 mg salt) per wk × 8 wk
- Severe falciparum malaria should be treated with parenteral therapy; mefloquine should not be used for the treatment of severe falciparum malaria, since there is no parenteral formulation. Options include quinine (as above), quinidine, artesunate and artemether.
- Exchange transfusion may be life saving in nonimmune patients with severe falciparum malaria and is indicated if parasitemia is > 30% or if parasitemia is > 10% and poor prognostic factors are present (i.e. elderly, schizonts in peripheral blood), there are severe systemic manifestations (i.e. cerebral malaria, pulmonary or renal failure) or therapeutic failure.
- Do not use doxycycline in pregnant women or in children < 8 years of age.
BOX 3. Control of Malaria
Vector Control
- Avoid mosquito-infested areas.
- Wear protective clothing during evening and nighttime hours.
- Use mosquito repellant (containing 30-35% DEET for adults or 6-10% DEET for children).
- Spray bedclothes and mosquito netting with the insect repellent permethrin.
- Unless absolutely necessary, pregnant women should not travel to P falciparum-endemic areas.
Prophylactic Measures
- Chloroquine remains the drug of choice in areas without known chloroquine resistance.
- Mefloquine is used in areas with known chloroquine-resistant strains.
- Doxycycline should be used when mefloquine cannot be taken, except by pregnant women, children < 8 y old, or those who are hypersensitive to doxycycline.
- Chloroquine & proguanil should be used only for patients who cannot take mefloquine or doxycycline
Emergency Self Treatment of Possible Malaria
- Individuals using chloroquine prophylaxis in areas where chloroquine-resistant strains may reside must have one or more treatment doses of Fansidar (25 mg pyrimethamine + 500 mg sulfadoxine/tablet) (adult dosage: 3 tablets PO as a single dose; pediatric dosage: 5-10 kg, 1/2 tablet; 11-20 kg, 1 tablet; 21-30 kg, 1 1/2 tablets; 31-45 kg, 2 tablets; > 45 kg, adult dose
BOX 4. Babesia-Associated Syndrome
More Common
- Asymptomatic
Less Common
- Mild infection: myalgia, low-grade fever with chills, fatigue, nausea, headache
- Severe infection (more common in splenectomized, elderly, or immuno-compromised patients): hemolytic anemia (with jaundice and dark urine), exacerbation of the above symptoms
BOX 5. Treatment of Babesiosis
Children
Adults
First Choice
- Clindamycin (20 mg/kg/d) for 7-10 d, PLUS quinine (25 mg/kg/d PO) taken for 7-10 d
- Clindamycin (300-600 mg every 6 h) PLUS quinine (650 mg PO every 6-8 h) taken for 7-10 d
BOX 6. Control of Babesiosis
Prophylactic Measure
- Avoid tick-infected areas
- Use appropriate clothing and tick repellants if avoidance is impractical
- Perform a body and scalp search for ticks on leaving infested areas