Definition
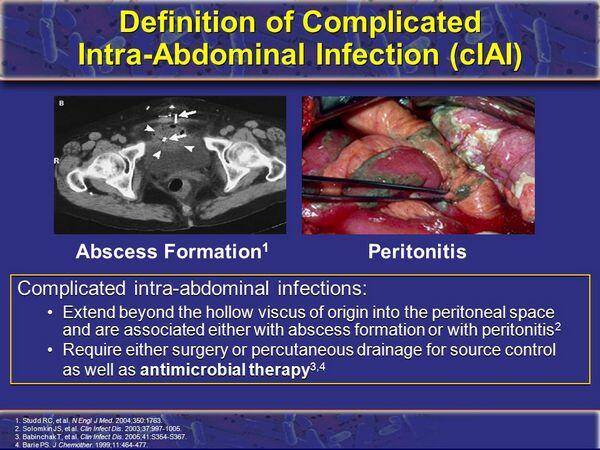
Intra-abdominal infections are those contained within the peritoneum or retroperitoneal space. Two general types of intra-abdominal infection are discussed throughout this chapter: peritonitis and abscess.
- Peritonitis is defined as the acute, inflammatory response of peritoneal lining to microorganisms, chemicals, irradiation, or foreign body injury. Peritonitis may be classified as either primary or secondary. With primary peritonitis, an intra-abdominal focus of disease may not be evident. In secondary peritonitis, a focal disease process is evident within the abdomen.
- An abscess is a purulent collection of fluid separated by a more or less well-defined wall from surrounding tissue. It usually contains necrotic debris, bacteria, and inflammatory cells.
Pathophysiology
- Table Causes of Bacterial Peritonitis summarizes many of the potential causes of bacterial peritonitis. The causes of intra-abdominal abscess somewhat overlap those of peritonitis and, in fact, both may occur sequentially or simultaneously. Appendicitis is the most frequent cause of abscess.
| TABLE. Causes of Bacterial Peritonitis | |
|
- Intra-abdominal infection results from entry of bacteria into the peritoneal or retroperitoneal spaces or from bacterial collections within intra-abdominal organs. When peritonitis results from peritoneal dialysis, skin surface flora are introduced via the peritoneal catheter.
- In secondary peritonitis, bacteria most often enter the peritoneum or retroperitoneum as a result of disruption of the integrity of the gastrointestinal tract caused by diseases or traumatic injuries.
- When bacteria become dispersed throughout the peritoneum, the inflammatory process involves the majority of the peritoneal lining.
- Peritonitis often results in mortality because of the effects on multiple organ systems. Fluid shifts and endotoxins may cause hypotension and shock. Fluid loss from the vasculature with generalized peritonitis is similar to that which occurs after a 50% second-degree skin burn.
- An abscess begins by the combined action of inflammatory cells (such as neutrophils), bacteria, fibrin, and other inflammatory components. A mature abscess may have a fibrinous capsule that isolates bacteria and the liquid core from antimicrobials and immunologic defenses.

Microbiology
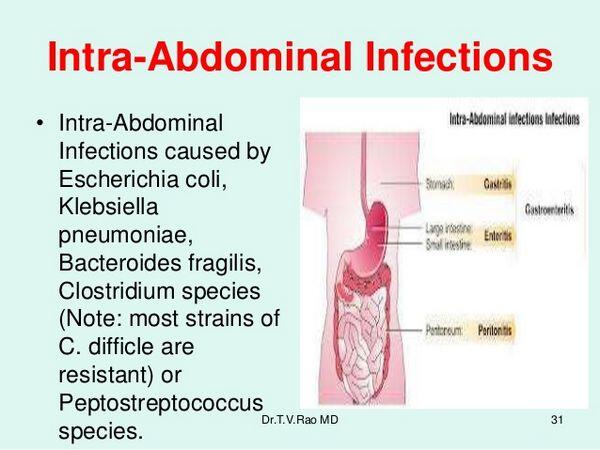
- Primary bacterial peritonitis is often caused by a single organism. In children, the pathogen is usually Streptococcus pneumoniae or a group A streptococcus. When peritonitis occurs in association with cirrhotic ascites, enteric organisms (such as Escherichia coli) are usually responsible.
- Peritonitis in patients undergoing peritoneal dialysis is most often caused by common skin organisms: Staphylococcus epidermidis, Staphylococcus aureus, streptococci, and diphtheroids.
- Secondary intra-abdominal infections are often polymicrobial. The mean number of isolates of microorganisms from infected intra-abdominal sites has ranged from 2.9 to 3.7, including an average of 1.3 to 1.6 aerobes and 1.7 to 2.1 anaerobes. The frequencies with which specific bacteria were isolated in intra-abdominal infections are given in Table Pathogens Isolated from Patients with Secondary Peritonitis.
- The combination of aerobic and anaerobic organisms appears to greatly increase pathogenicity. In intra-abdominal infections, facultative bacteria may provide an environment conducive to the growth of anaerobic bacteria.
| TABLE. Pathogens Isolated from Patients with Secondary Peritonitis | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
- Aerobic enteric bacteria and anaerobic bacteria are both pathogens in intra-abdominal infection. Aerobic bacteria, particularly E. coli, appear responsible for the early mortality from peritonitis, whereas anaerobic bacteria are major pathogens in abscesses, with Bacteroides fragilis predominating.
- The role of Enterococcus as a pathogen is not clear. Enterococcal infection occurs more commonly in postoperative peritonitis, in the presence of specific risk factors indicating failure of the host defenses, or with the use of broad-spectrum antibiotics.
Clinical presentation
- Intra-abdominal infections have a wide spectrum of clinical features often depending on the specific disease process, the location and the magnitude of bacterial contamination, and concurrent host factors. Patients with primary and secondary peritonitis present quite differently (Table Clinical Presentation of Peritonitis).
- If peritonitis continues untreated, the patient may go into hypovolemic shock from fluid loss into the peritoneum, bowel wall, and lumen. This may be accompanied by generalized sepsis.
Desired outcome
- The goals of treatment are the correction of intra-abdominal disease processes or injuries that have caused infection and the drainage of collections of purulent material (e.g., abscess).
- A secondary objective is to achieve resolution of infection without major organ system complications or adverse treatment effects.
Treatment
General principles
- The three major modalities for the treatment of intra-abdominal infection are prompt drainage, support of vital functions, and appropriate antimicrobial therapy to treat infection not removed by surgery.
- Antimicrobials are an important adjunct to surgical procedures in the treatment of intra-abdominal infections; however, the use of antimicrobial agents without surgical intervention is usually inadequate. For some specific situations (e.g., most cases of primary peritonitis), drainage procedures may not be required, and antimicrobial agents become the mainstay of therapy.
- With generalized peritonitis, large volumes of intravenous fluids are required to restore vascular volume and improve cardiovascular function.
Nonpharmacologic treatment
- Secondary peritonitis requires surgical correction of the underlying pathology. Drainage of the purulent material, either by open surgical procedure or drained percutaneously, is the critical element in the management of an intra-abdominal abscess.
- Aggressive fluid repletion and management are required for the purposes of achieving or maintaining proper intravascular volumes and adequate urine output and correcting acidosis.
- In the initial hour of treatment, a large volume of intravenous solution (lactated Ringers) may need to be administered to restore intravascular volume. This may be followed by up to 1 L/h until fluid balance is restored in a few hours.
| TABLE. Clinical Presentation of Peritonitis | ||||
|
- In patients with significant blood loss (hematocrit of 25%), blood should be given. This is generally in the form of packed red blood cells.
Pharmacologic therapy
- The goals of antimicrobial therapy are to control bacteremia and to establish the metastatic foci of infection, to reduce suppurative complications after bacterial contamination, and to prevent local spread of existing infection.
| TABLE. Likely Intra-abdominal Pathogens | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
- An empiric antimicrobial regimen should be started as soon as the presence of intra-abdominal infection is suspected on the basis of likely pathogens.
- Likely pathogens, those against which antimicrobial agents should be directed, are listed in Table Likely Intra-abdominal Pathogens.
- Table Guidelines for Initial Antimicrobial Agents for Intra-abdominal Infections presents recommended and alternative regimens for selected situations. These are general guidelines, not rules, because there are many factors that cannot be incorporated into such a table.
Recommendations
- Most patients with severe intra-abdominal infections (where there is generalized peritonitis or sepsis should be placed on a β-lactam or β- lactamase inhibitor combination, or carbapenem (imipenem, ertapenem or meropenem). Combinations of an aminoglycoside with an antianaerobic agent such as clindamycin or metronidazole may be used, but some authorities consider such combinations to be obsolete.
- The selection of a specific agent or combination should be based on culture and susceptibility data for peritonitis that occurs from CPD. If microbiologic data are unavailable, empiric therapy should be initiated.
- For established intra-abdominal infections, most patients are adequately treated with 5 to 7 days of antimicrobial therapy.
- Patients with peritonitis who are undergoing CPD may receive parenteral as well as intraperitoneal antimicrobial agents. Intraperitoneal antimicrobial agents alone are often sufficient, unless severe infection is present. Recommended concentrations of antimicrobial agents for intraperitoneal irrigation solutions are 8 mg/L for gentamicin and tobramycin, 1 to 3 mg/L for clindamycin, 50,000 U/L for penicillin G, 125 mg/L for cephalosporins, 100 to 150 mg/L for ticarcillin or carbenicillin, 50 mg/L for ampicillin, 100 mg/L for methicillin, 30 mg/L for vancomycin, and 3 mg/L for amphotericin B.
- The usual duration of therapy for peritonitis associated with CPD is 10 to 14 days but may extend to 3 weeks. Antimicrobial therapy should be continued until dialysate fluid is clear, cultures are negative for 2 to 3 days, and the patient is asymptomatic.
| TABLE. Guidelines for Initial Antimicrobial Agents for Intra-abdominal Infections | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- Antianaerobic cephalosporins or extended-spectrum penicillins are effective in preventing most infectious complications after acute bacterial contamination, such as with abdominal trauma where gastrointestinal contents enter the peritoneum, and when the patient is seen soon after injury (within 2 hours) and surgical measures are instituted promptly.
- Acute intra-abdominal contamination, such as after a traumatic injury, may be treated with a short course (24 hours). For established infections (peritonitis or intra-abdominal abscess), an antimicrobial course of at least 7 days is justified.
Evaluation of therapeutic outcomes
- The patient should be continually reassessed to determine the success or failure of therapies.
- Unsatisfactory outcomes in patients with intra-abdominal infections may result from complications that arise in other organ systems. A complication commonly associated with mortality after intra-abdominal infection is pneumonia.
- Once antimicrobials are initiated and other important therapies described earlier are used, most patients should show improvement within 2 to 3 days. Usually, temperature will return to near normal, vital signs should stabilize, and the patient should not appear in distress, with the exception of recognized discomfort and pain from incisions, drains, and nasogastric tube.
- At 24 to 48 hours, aerobic bacterial culture results should return. If a suspected pathogen is not sensitive to the antimicrobial agents being given, the regimen should be changed if the patient has not shown sufficient progress.
- If the isolated pathogen is extremely sensitive to one antimicrobial, and the patient is progressing well, concurrent antimicrobial therapy may often be discontinued.
- With present anaerobic culturing techniques and the slow growth of these organisms, anaerobes are often not identified until 4 to 7 days after culture, and sensitivity information is difficult to obtain. For this reason, there are usually few data with which to alter the antianaerobic component of the antimicrobial regimen.
- Superinfection in patients being treated for intra-abdominal infection is often due to Candida, but enterococci or opportunistic gram-negative bacilli such as Pseudomonas or Serratia may be involved.
- Treatment regimens for intra-abdominal infection can be judged successful if the patient recovers from the infection without recurrent peritonitis or intra-abdominal abscess and without the need for additional antimicrobials. A regimen can be considered unsuccessful if a significant adverse drug reaction occurs, if reoperation is necessary, or if patient improvement is delayed beyond 1 or 2 weeks.




