Invasive pulmonary aspergillosis in the immunocompromised host is among the most serious manifestations of disease caused by Aspergillus spp.
Key risk factors for invasive aspergillosis include neutropenia, especially profound neutropenia (< 100 neutrophils/mL) and prolonged neutropenia (> 12 days); prolonged high-dose corticosteroid therapy, graft-versus-host disease after bone marrow transplantation, acute rejection after solid-organ transplantation, cytomegalovirus disease after transplantation, advanced AIDS, and poorly controlled diabetes mellitus.
On very rare occasions, invasive pulmonary aspergillosis may occur in previously healthy adults or in patients with alcoholic liver disease.
Chronic necrotizing aspergillosis is an indolent form of invasive pulmonary aspergillosis that occurs in patients who are less profoundly immunosuppressed than those with the risk factors cited above.
Clinical Findings
Signs and Symptoms
Severely immunosuppressed patients with invasive aspergillosis may be completely asymptomatic when the disease is first suspected. The initial clue may only be a positive sputum culture or an abnormal chest x-ray. Nonproductive cough, dyspnea, and chest pain are common among patients with symptoms. Pleuritic chest pain is occasionally seen. Fever is usually present but may be suppressed by corticosteroid therapy. Hemoptysis may develop as a consequence of angioinvasion and, in the appropriate host, should raise the suspicion of invasive pulmonary aspergillosis.
Laboratory Findings
Hypoxemia is often present in patients with extensive pulmonary involvement. Other laboratory abnormalities are nonspecific. Because of the immunodeficient state, serum antibodies to Aspergillus spp. are usually negative. The detection of circulating Aspergillus antigen is a promising investigational test but is not yet available commercially. Cultures of the sputum or respiratory secretions are often positive. Unfortunately, recovery of Aspergillus isolates from the sputum of otherwise healthy people is occasionally seen so it may be difficult and a challenge to distinguish airway colonization from positive cultures caused by invasive disease. Furthermore, 20-30%of patients with invasive pulmonary aspergillosis will have negative sputum cultures unless serial sampling is done. Positive blood cultures for Aspergillus spp. are exceedingly rare.
Imaging
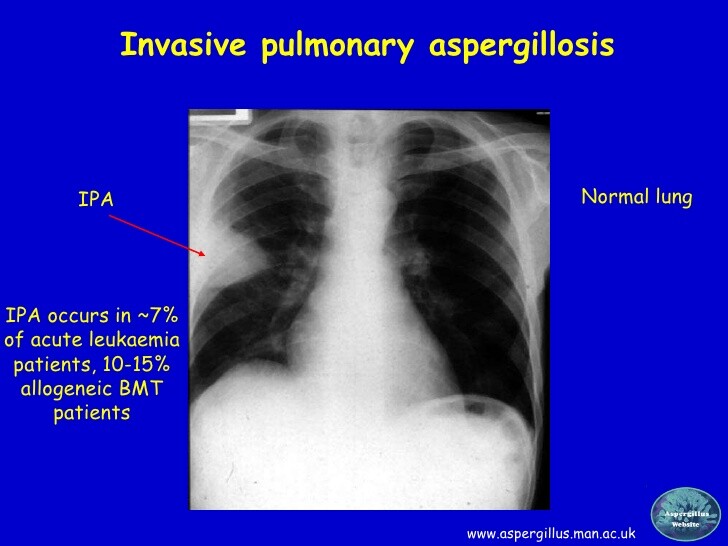
The chest x-ray findings in patients with invasive pulmonary aspergillosis vary considerably. Focal pneumonitis, diffuse patchy disease, mononodular and multinodular densities, and pleural-based infiltrates may be seen. Rounded nodular infiltrates are the most common finding. Serial chest x-rays may show rapid progression of pulmonary involvement, and cavitation in this setting is common. Computed-tomography (CT) imaging of the chest may show a characteristic halo sign around nodular infiltrates that are highly suggestive of angioinvasive Aspergillus infection.
Differential Diagnosis
The differential diagnosis of a new infiltrative lung process in an immunosuppressed patient is very broad. Other fungi including Pseudallescheria boydii and the order Mucorales can cause an identical syndrome. Nocardia lung infection can be similar in presentation. Mycobacterial infection is less likely but must be considered. Bacterial infection including that caused by Staphylococcus aureus and Pseudomonas aeruginosa is possible. Rhodococcus equi is a rare but emerging pathogen that presents this way, especially if cavitation is present.
Complications
The propensity for angioinvasion leads to hematogenous dissemination in undiagnosed or untreated cases. Virtually any organ may be involved (see disseminated aspergillosis).
Diagnosis
The diagnosis of invasive pulmonary aspergillosis in the immunosuppressed patient is a significant challenge. A definitive diagnosis is established when tissue specimens demonstrate invasive fungal elements; however, a positive sputum specimen in a high-risk patient and imaging evidence consistent with Aspergillus infection should be regarded as presumptive invasive pulmonary aspergillosis and justification for empiric antifungal therapy. In patients with negative cultures or in whom sputum cultures cannot be obtained, bronchoscopy and bronchoalveolar lavage may help establish a microbiologic diagnosis. Bronchoscopic transbronchial biopsy may yield a false negative result due to the patchy nature of pulmonary aspergillosis and to a sampling error of the biopsy. In patients with pleural-based nodular disease, percutaneous lung biopsy may be considered. Pneumothorax and bleeding are potential complications of this approach. Thorascopic or open lung biopsy is the most effective method to establish the diagnosis.
Treatment
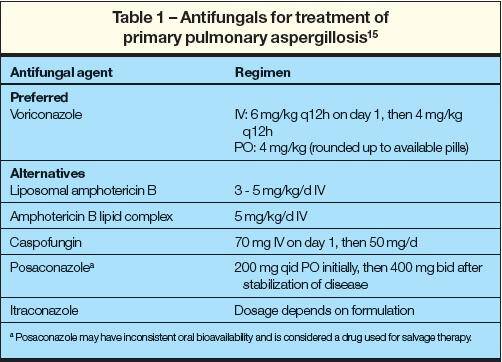
Invasive pulmonary aspergillosis may be a rapidly progressive infection and prompt institution of antifungal therapy is critical once the diagnosis is suspected or confirmed (see Box 2). Amphotericin B remains the drug of choice. Treatment failures are common.
Alternatives to amphotericin B include lipid amphotericin B preparations. These preparations are considerably less nephrotoxic than standard amphotericin B and appear to be equally efficacious.
Unfortunately, these new lipid preparations are currently exceedingly expensive. Itraconazole is an oral azole with activity against Aspergillus spp. Its use should be reserved for patients who are not severely immunosuppressed, who have an indolent or chronic infection, or who are intolerant of amphotericin B therapy. The optimal duration of antifungal therapy is not known, but the cumulative total dose is = 2 g of amphotericin B. In patients with a single focus of pulmonary infection, resection should be considered.
Prognosis
The prognosis for invasive pulmonary aspergillosis is generally poor. Cure or improvement on therapy is seen in ~ 50% of cases. Response is more likely if neutropenia recovers and immunosuppression is reduced or reversed.
Prevention & Control
Because invasive aspergillosis in the immunosuppressed or neutropenic patient is difficult to diagnosis and treat, much attention has been focused on prevention as a way of reducing the frequency of infection (Box 3). Two general approaches have been used: reduction of environmental exposure and prophylactic antifungal therapy. Aspergillus spp. are an ubiquitous component of dust, building material, and organic debris.
Reducing exposure to at-risk hospitalized patients can be achieved in several ways. Potted plants should be removed from the environment adjacent to at-risk patients. Certain foods, such as cereal and spices, have been found to be contaminated with Aspergillus spores and should not be offered to hospitalized patients that are at risk for aspergillosis. Construction in and adjacent to hospitals has been associated with outbreaks of nosocomial aspergillosis.
Patients should not be treated in areas of the hospital where construction or remodeling is occurring. If a patient must be transported through areas of construction within the hospital, an efficient mask should be worn by the patient. Efforts should be made to seal areas undergoing construction or remodeling to prevent contamination of the air in patient areas.
HEPA filtration of air has been shown to significantly reduce or eliminate Aspergillus spores. Patient rooms with laminar airflow and HEPA filtration appear to be effective in reducing the risk of exposure in the hospital. Unfortunately, the construction and maintenance of HEPA-filtered facilities are very expensive and not available to all patients. Other strategies that have been used include high air exchange rates, surveillance air sampling, positive pressure in the patient’s room and immediate environment, and regular filter changing of point-of-use air filtration systems.
The use of intravenous amphotericin B for prophylaxis against Aspergillus spp. has been investigated. When given prophylactically at a dose of 1 mg/kg/d, the toxicity is prohibitively high. When used prophylactically at lower doses of 0.1-0.25 mg/kg/d, the toxicity is less, but in most studies, this dosage was insufficient to prevent aspergillosis. The lipid formulations of amphotericin B are attractive for prophylaxis alternatives; however, the high cost of these preparations limit their widespread use.
Among the oral azoles, only itraconazole has sufficient activity against Aspergillus to be considered as a viable prophylactic agent. There are limited data confirming its efficacy in preventing aspergillosis in high-risk patients. Moreover, the absorption of itraconazole from the gastrointestinal tract may be inadequate in these patients.
Use of intranasal installation of amphotericin B or aerosolization of amphotericin B was an effective prophylaxis in several small pilot studies. More data are necessary regarding optimal dose, frequency, and duration before this approach can be widely recommended for prophylaxis.
Links
http://err.ersjournals.com/content/20/121/156
http://erj.ersjournals.com/content/19/4/743
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4318821/
http://www.mayoclinic.org/diseases-conditions/aspergillosis/basics/symptoms/con-20030330