Essentials of Diagnosis
- Predisposing factors include travel to the dry desert climates found in the southwestern United States and exposure to dust.
- The commonest source of infection is dust inhalation in the southwestern United States.
- The commonest infection is pneumonia.
- Key laboratory findings include growth of the fungus and complement fixing (CF) antigen detection by immunodiffusion.
General Considerations
Coccidioidomycosis was first described as a disease a little more than a century ago in Buenos Aires, Argentina. It was in San Francisco that the organism causing the clinical disease was given its name, Coccidioides immitis. Many diseases were later found to be caused by this organism, including San Joaquin Valley Fever.
Epidemiology
Coccidioidomycosis is not a new disease. Recently, however, there has been an increase in the number of cases reported in the United States. C immitis is endemic to the southwestern United States, found primarily in southern California, southern Nevada, southern New Mexico, and Arizona. Other endemic areas have similar dry desert climates such as Mexico and South and Central America. This disease is no longer limited to residents of these areas. As travel increases, coccidioidomycosis must always be considered when the clinical picture fits. This fact emphasizes the importance of obtaining a good travel history.
Exposure to dust affects the incidence of coccidioidomycosis. Incidence is highest in the summer and fall when the soil is dry and dusty. Earthquakes and dust storms also increase incidence. In the United States an estimated 100,000 infections occur annually.
Infection doesn’t necessarily mean clinical manifestation of disease. Infection has been studied among soldiers stationed in the San Joaquin Valley during WWII. The soldiers were skin tested and questioned about illnesses. Of all reported infections, 60% were found to be asymptomatic, another 35% were self-limited, and 5% resulted in disseminated disease.
Microbiology and Pathogenesis
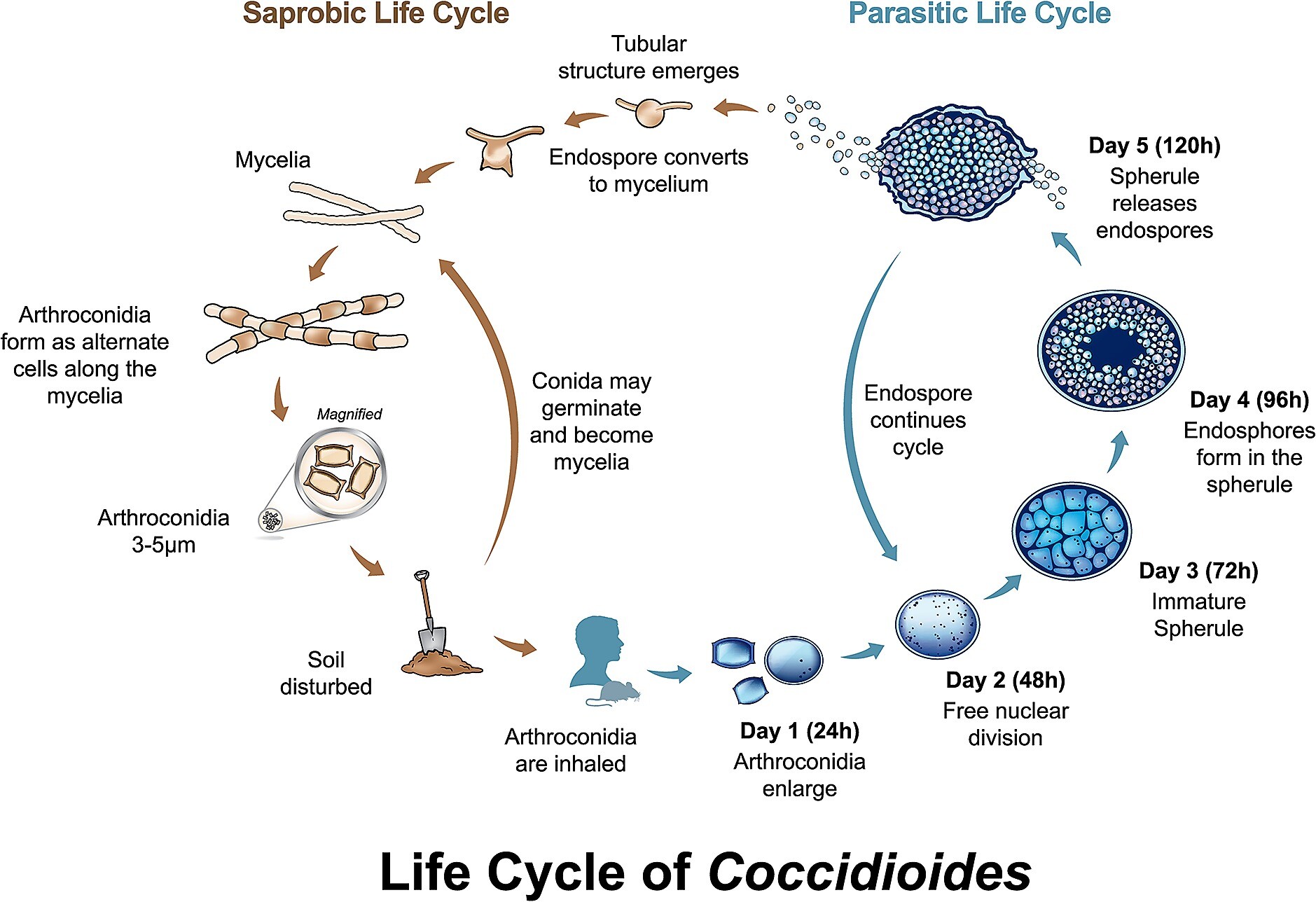
C immitis is a fungus that lives in the soil. As the fungus matures it forms alternate cells along a hypha, called arthroconidia. This stage of the fungus can become airborne and be inhaled by a human host, which causes infection. A very small number of arthroconidia are needed to cause infection. Once the host is infected, multiple divisions of the inhaled arthroconidia occur in the lung, forming spherules and then thousands of endospores. Each endospore can repeat the development of a spherule. Thus, a cyclic process occurs, and multiplication is rapid. The immunologic response to C immitis is primarily cellular. Functioning T lymphocytes are essential to control infection.
Clinicial Findings
Signs and Symptoms
In symptomatic disease, first manifestations occur 1-3 weeks after initial exposure. The symptoms are clinically nonspecific and include cough with scant sputum production, fever, headache, and pleuritic chest pain in the majority of cases (Table 1).
A skin rash is common with primary infection. Both erythema nodosum and erythema multiforme are referred to as “specific erythemas” of coccidioidomycosis. These erythemas are associated with an intense immunologic response and with a good prognosis. People who exhibit these erythemas will likely not progress to disseminated disease.
The most common clinical manifestation of disease is pneumonia (Box 1). Most are self-resolved and likely go undiagnosed. Pulmonary complications occur infrequently and include asymptomatic nodules or thin-walled cavities, slow resolving pneumonia, or chronic lung infection.
Nodules occur in 5-7% of patients and are usually solitary and asymptomatic. The morbidity associated with these lesions occurs during surgical resection of the nodules to exclude cancer. More recently percutaneous fine-needle biopsy has been used for diagnosis.
Cavities occur in younger patients, are most frequently asymptomatic, and are usually self-resolved, disappearing within 2 years of appearance. Complications, however, can occur and are usually associated with air fluid levels or infiltrates surrounding the cavity. Infection rarely spreads to other areas of the lung. If rupture of the cavity occurs, surgical repair is required.
Disseminated disease is unusual. Fewer than 5% of infected persons experience disseminated disease. Extrapulmonary disease includes granulomatous skin lesions or subcutaneous abscess, septic arthritis, osteomyelitis, and meningitis. Risk factors for extrapulmonary disease have been found to be male sex, pregnant women, immunosuppression, nonwhites (especially Filipinos and blacks), and possibly age (the very old or young).
Meningitis occurs in one-third to one-half of all patients with coccidioidal dissemination. The clinical symptoms are not specific to C immitis infection and include headache, vomiting, nuchal rigidity, confusion, and diplopia. Lumbar puncture is essential to diagnosis. The cerebrospinal fluid reveals a pleocytosis with a predominance of mononuclear cells. Most have an eosinophilia as well. Usually the glucose is low, and the protein elevated. The cerebrospinal fluid should be sent both for culture and serologic tests (complement-fixing antigen).
Coccidioidal meningitis should be suspected in patients with the clinical syndrome and a recent coccidioidal infection. Meningitis usually occurs within 6 months of initial infection; however, meningitis can occur late if immunosuppressed. Early diagnosis is important, and, if left untreated, coccidioidal meningitis is fatal. Even with diagnosis, treatment is difficult.
Laboratory Findings
Coccidioidal infections should be considered in patients with a pulmonary syndrome and who live in or have a history of travel to endemic areas. As stated earlier, most of these infections are self-limited, and physicians living outside endemic areas will likely see only complicated cases.
The vast majority of cases are asymptomatic and will be identified by a positive skin test only. Positive skin tests are neither definitive nor helpful in establishing the diagnosis of coccidioidomycosis, because of the high number of asymptomatic infections. Definitive diagnosis is established via culture of the organism or antibody or antigen detection. Antigen detection kits are not currently commercially available.
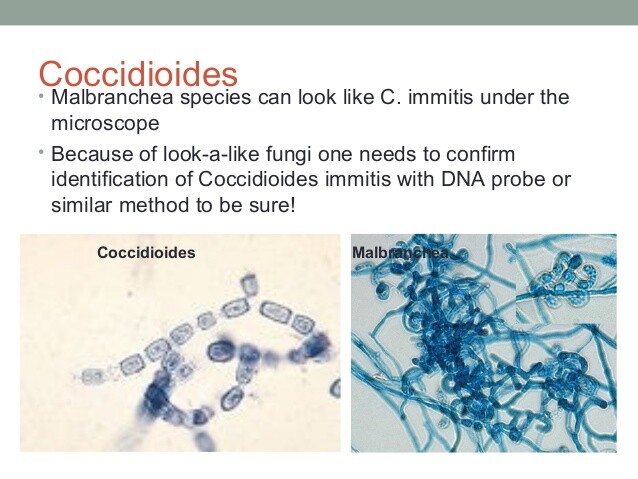
C immitis readily grows in the laboratory and is detectable as early as 2-5 days after inoculation. The fungal growth is further evaluated via a species-specific DNA probe. Results are available the day growth is detected. It is important to notify the laboratory of your suspicions, because laboratory identification may be dangerous to the lab technician. Arthrospores readily become airborne and may be inhaled.
There are two coccidioidal antigens that are targeted for antibody detection, the tube precipitin reading (TP) antigen and the CF antigen. Antibody detection kits are not available, and instead laboratories use immunodiffusion tests. Both tests are reported qualitatively; if immunodiffusion CF is positive, a follow-up quantitative test is necessary. The concentration of CF antibodies is proportional to the extent of the disease. Ordering physicians must be aware of false-negative results in detecting anticoccidioidal antibodies; these results are most prevalent in early primary infection or in immunosuppressed patients.
Differential Diagnosis
Coccidioidal infections are suspected in patients with pulmonary symptoms and the appropriate travel or residence in an endemic area. Bacterial or other fungal pneumonias should be included in the differential.
Complications
Most coccidioidal infections are self-resolved. Pulmonary complications can occur and include asymptomatic nodules, slowly resolving pneumonia, or chronic lung infection. The most serious complication includes disseminated disease and can affect any organ system.
Treatment
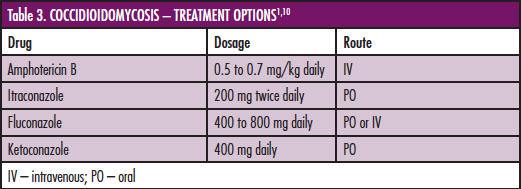
In most cases the decision to treat is the real question. Recall that even for patients who develop symptoms of initial infection, the disease is usually self-limited. In patients in whom treatment is indicated, coccidioidomycosis is very difficult to cure. Treatment for initial infection is indicated only for patients with deficiencies in T-cell immunity. A physician may decide to treat a severe pulmonary infection, but there is no expert consensus. Treatment is indicated for chronic pulmonary infection and extrapulmonary dissemination. Accepted-standard-of-care therapies include amphotericin B and oral azole antifungal agents (Boxes 2 and 3). Fluconazole and itraconazole have been shown to have similar success rates. Length of treatment is controversial, because relapse is common.
Treatment may be lifelong. Most physicians will treat pulmonary and disseminated disease for 12-18 months. It has been found that negative serial coccidioidin skin tests and very high CF antibody titers are independently associated with increased risk of relapse. Rising CF titers after completion of therapy are an indication of relapse and warrant retreatment. Patients with meningitis are likely never cured and require indefinite treatment. Only fluconazole and amphotericin B have been shown to be effective therapies. If amphotericin B is used, both parenteral and intrathecal therapy is indicated.
Prevention
There is no vaccine available against C immitis infection. General prevention includes avoiding exposure to the fungus in its natural setting and decreasing the amount of airborne dust. Environmental measures including paving dirt roads and planting grass may be implemented.
Table 1. Clinical features of primary coccidioidal infection.1
Symptom
Frequency (%)
Cough
89
Fever
82
Chest pain
70
Headache
74
Shortness of breath
63
Malaise
59
Myalgias
52
Rash
52
BOX 1. Coccidioidal Infection
Children
Adults
More Common
- Mild upper respiratory infection
- Subacute, self-limited pneumonia
- Mild upper respiratory infection
- Self-limited pneumonia
Less Common
- Complicated pulmonary infection
- Extrapulmonary disease
- Complicated pulmonary infection
- Extrapulmonary disease
BOX 2. Treatment of Coccidioidal Infection in Adults
Site
Primary
Alternative
Pulmonary and Extrapulmonary
Fluconazole, 400-800 mg orally daily (duration 12-18 months)
Itraconazole 200 mg orally twice a day (duration 12-18 months) OR
Amphotericin B 0.6-1.0 mg/kg/d IV × 7 d then 0.8 mg/kg/day total dose 2.5 gms or more)
Meningitis
Fluconazole 400-600 mg orally daily indefinitely.
Amphotericin B IV as above and intrathecal 0.1-0.3 md daily
BOX 3. Indications for Treatment of Coccidioidal Infection
Site/Indications
Treatment
Fever lasting longer than 1 month
Extensive or progressive pulmonary disease
Immunosuppressed patients
HIV-infected patients
Pregnant patients
Amphotericin B is used as initial drug of choice because of rapid improvement in symptoms, followed by oral azoles.
Amphotericin B: 0.6-1.0 mg/kg/day for 7 days, then 0.8 mg/kg/every other day. Total dose 2.5 g.
Oral azoles: Itraconazole 200 mg twice a day OR fluconazole 400 mg/day for 3-12 months.
Disseminated disease
Amphotericin B, 0.5-0.7 mg/kg/day. Total dose, usually 15-45 mg/kg.
Total dose not clearly defined.
Links
https://en.wikipedia.org/wiki/Coccidioides
http://www.the-dermatologist.com/content/treating-rare-fungal-infections-coccidioidomycosis