Definition
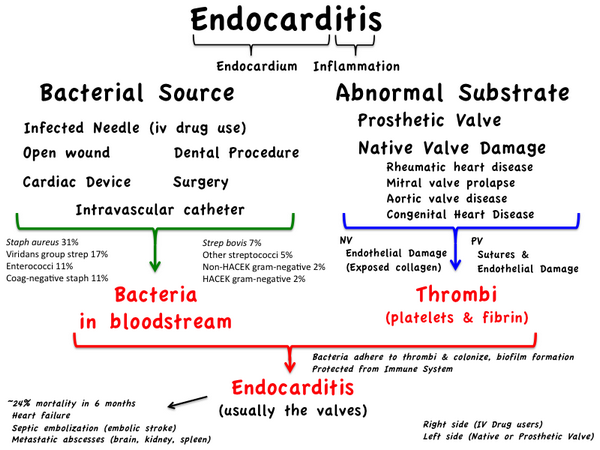
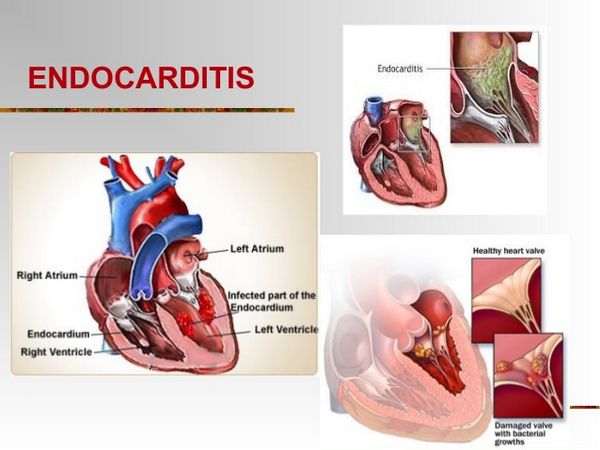
Endocarditis is an inflammation of the endocardium, the membrane lining the chambers of the heart and covering the cusps of the heart valves. Infective endocarditis refers to infection of the heart valves by microorganisms. Endocarditis is often referred to as either acute or subacute depending on the clinical presentation. Acute bacterial endocarditis is a fulminating infection associated with high fevers, systemic toxicity, and death within a few days to weeks if untreated. Subacute infectious endocarditis is a more indolent infection, usually occurring in a setting of prior valvular heart disease.
Pathophysiology
- Most patients with Infective endocarditis have risk factors, such as preexisting cardiac valve abnormalities.
- Most types of structural heart disease resulting in turbulence of blood flow will increase the risk for Infective endocarditis. Some of the most important include the following:
- Presence of a prosthetic valve (400-fold increased risk)
- Previous endocarditis (400-fold increased risk)
- Complex cyanotic congenital heart disease (e.g., single ventricle states)
- Surgically constructed systemic pulmonary shunts or conduits
- Acquired valvular dysfunction (e.g., rheumatic heart disease)
- Hypertrophic cardiomyopathy
- Mitral valve prolapse with regurgitation
- Intravenous drug abuse
- Three groups of organisms cause most cases of Infective endocarditis: streptococci (55% to 62%), staphylococci (25% to 35%), and enterococci (5% to 18%) (Table Etiologic Organisms in Infective Endocarditis).
Clinical presentation
- The clinical presentation of patients with Infective endocarditis is highly variable and nonspecific (Table Clinical Presentation of Infective Endocarditis).
| TABLE. Etiologic Organisms in Infective Endocarditis |
| Agent |
Percentage of Cases |
| Streptococci |
55-62 |
| Viridans streptococci |
30-40 |
| Other streptococci |
15-25 |
| Staphylococci |
20-35 |
| Coagulase-positive |
10-27 |
| Coagulase-negative |
1-3 |
| Enterococci |
5-18 |
| Gram-negative aerobic bacilli |
1.5-13 |
| Fungi |
2-4 |
| Miscellaneous bacteria |
<5 |
| Mixed Infections |
1-2 |
| «Culture negative» |
<5-24 |
|
| TABLE. Clinical Presentation of Infective Endocarditis |
| General The clinical presentation of Infective endocarditis is highly variable and nonspecific. Symptoms The patient may complain of fever, chills, weakness, dyspnea, night sweats, weight loss, and/or malaise. Signs Fever is common as well as a heart murmur (sometimes new or changing). The patient may or may not have embolic phenomenon, spleanomegaly, or skin manifestations (e.g., Osler nodes, Janeway lesions). Laboratory Tests The patient’s white blood cell count may be normal or only slightly elevated. Nonspecific findings include anemia (normocytic, normochromic), thrombocytopenia, an elevated erythrocyte sedimentation rate or C-reactive protein, and altered urinary analysis (proteinuria/microscopic hematuria). The hallmark laboratory finding is continuous bacteremia; three sets of blood cultures should be collected over 24 h. Other Diagnostic Tests An electrocardiogram, chest radiograph, and echocardiogram are commonly performed. Echocardiography to determine the presence of valvular vegetations plays a key role in the diagnosis of Infective endocarditis; it should be performed in all suspected cases. |
|
- Important clinical signs, especially prevalent in subacute illness, may include the following peripheral manifestations («stigmata») of endocarditis:
- Osler nodes
- Janeway lesions
- Splinter hemorrhages
- Petechiae
- Clubbing of the fingers
- Roth spots
- Emboli
- Without appropriate antimicrobial therapy and surgery Infective endocarditis is usually fatal. With proper management, recovery can be expected in most patients.
- Factors associated with increased mortality include the following:
- Congestive heart failure
- Culture-negative endocarditis
- Endocarditis caused by resistant organisms such as fungi and gram-negative bacteria
- Left-sided endocarditis caused by Staphylococcus aureus
- Prosthetic valve endocarditis
Laboratory and diagnostic findings
- The hallmark of Infective endocarditis is a continuous bacteremia caused by shedding of bacteria from the vegetation into the bloodstream. More than 95% of patients with Infective endocarditis have a positive blood culture when three samples are obtained during a 24-hour period.
- Transesophageal echocardiography is important in identifying and localizing valvular lesions in patients suspected of having Infective endocarditis. Transesophageal echocardiography is more sensitive for detecting vegetations (90% to 100%), compared to transthoracic echocardiography (58% to 63%).
- The Modified Duke criteria, encompassing major findings of persistent bacteremia and echocardiographic findings and other minor findings, are used to categorize patients as «definite Infective endocarditis» or «possible Infective endocarditis»
Desired outcome
- Relieve the signs and symptoms of disease
- Decrease morbidity and mortality associated with infection
- Eradicate the causative organism with minimal drug exposure
- Provide cost-effective antimicrobial therapy
Prevent Infective endocarditis in high-risk patients with appropriate antimicrobials
Prevention of Endocarditis
- Antimicrobial prophylaxis is used to prevent Infective endocarditis in patients believed to be at high risk.
- The use of antimicrobials for this purpose requires consideration of the types of patients who are at risk; the procedures causing bacteremia; the organisms that are likely to cause endocarditis; and the pharmacokinetics, spectrum, cost, and ease of administration of available agents. The objective of prophylaxis is to diminish the likelihood of Infective endocarditis in high-risk individuals who are undergoing procedures that cause transient bacteremia.
Patients at risk
- Patients with certain cardiac lesions, particularly those with a history of rheumatic heart disease and prosthetic heart valves, are at risk for developing endocarditis. However, only 15% to 25% of patients who develop Infective endocarditis are in a definable high-risk category, and only a small proportion of high-risk patients will develop Infective endocarditis if prophylaxis is not given.
Procedures causing bacteremia
For dental procedures of the gums and oral structures that cause bleeding, viridans streptococci frequently cause bacteremia, whereas instrumentation and surgery of the gastrointestinal and genitourinary tracts more often result in enterococcal bacteremia.
| TABLE. Prophylactic Regimens for Genitourinary Gastrointestinal (Excluding Esophageal) Procedures |
| Situation |
Agenta |
Regimenb |
| High-risk patients |
Ampicillin plus gentamicin |
Adults: Ampicillin 2 g intramuscularly (intramuscularly) or intravenously (intravenous) plus gentamicin 1.5 mg/kg (not to exceed 120 mg) within 30 min of starting the procedure; 6 h later, ampicillin 1 g intramuscularly/intravenous or amoxicillin 1 g orally. |
| Children: Ampicillin 50 mg/kg intramuscularly or intravenous (not to exceed 2 g) plus gentamicin 1.5 mg/kg within 30 min of starting the procedure; 6 h later, ampicillin 25 mg/kg intramuscularly/intravenous or amoxicillin 25 mg/kg orally. |
| High-risk patients allergic to ampicillin/amoxicillin |
Vancomycin plus gentamicin |
Adults: Vancomycin 1 g intravenous over 1-2 h plus gentamicin 1.5 mg/kg intravenous/intramuscularly (not to exceed 120 mg); complete injection/infusion within 30 min of starting the procedure. |
| Children: Vancomycin 20 mg/kg intravenous over 1-2 h plus gentamicin 1.5 mg/kg intravenous/intramuscularly; complete injection or infusion within 30 min of starting the procedure. |
| Moderate-risk patients |
Amoxicillin or Ampicillin |
Adults: Amoxicillin 2 g orally 1 h before procedure, or ampicillin 2 g intramuscularly/intravenous within 30 min of starting the procedure. |
| Children: Amoxicillin 50 mg/kg orally 1 h before procedure, or ampicillin 50 mg/kg intramuscularly/intravenous within 30 min of starting the procedure. |
| Moderate-risk patients allergic to ampicillin/amoxicillin |
Vancomycin |
Adults: Vancomycin 1 g intravenous over 1-2 h; complete infusion within 30 min of starting the procedure. |
| Children: Vancomycin 20 mg/kg intravenous over 1-2 h; complete infusion within 30 min of starting the procedure. |
| aTotal children’s dose should not exceed adult dose. bNo second dose of vancomycin or gentamicin is recommended. |
|
Antibiotic regimens
- A 2-g dose of amoxicillin is recommended for adult patients at risk, given 60 minutes prior to undergoing procedures associated with bacteremia. For penicillin-allergic patients or those undergoing gastrointestinal surgery, alternative prophylaxis is recommended.