Epidemiology
Infective endocarditis is a microbial infection of the endothelial lining of the heart. The characteristic lesion is a vegetation (a mass comprised of fibrin, platelets, microorganisms and their product or products on a valve leaflet). Multiple valves may be involved, as may any part of the endothelium of the heart. The incidence of endocarditis ranges from 17 to 39 cases per million person-years.
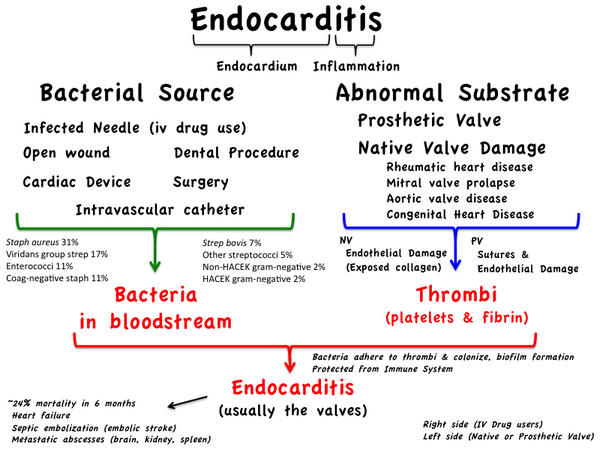
There have been major changes in the epidemiology of endocarditis. The age of patients affected has increased considerably. In 1945, about 10% of patients with Infective endocarditis were over 60 years of age (Lerner and Weinstein 1966), compared with 55% in 1977. The second major change in the epidemiology of endocarditis is the increase in the incidence of prosthetic valve endocarditis, which accounts for almost half of the cases of endocarditis treated at tertiary-care medical centers. prosthetic valve endocarditis is divided into two categories, early (EPVE), which develops less than 60 days after surgery, and late (LPVE), which develops more than 60 days after surgery. Endocarditis complicates 0.98% to 4.4% of prosthetic valves.
The underlying cardiac lesions in native valve endocarditis have also changed in the modern era. In the preantibiotic era, rheumatic heart disease was the most common lesion. In a recent series of 63 patients with native valve endocarditis, the underlying cardiac lesions were as follows: mitral valve prolapse, 29%; no underlying disease, 27%; degenerative lesions of the aortic or mitral valve, 21%; congenital heart disease, 13%; rheumatic heart disease, 6%. In cases of mitral valve prolapse, the risk of infection is significantly higher among those with redundancy (the classic form) compared with those without leaflet thickening (the nonclassic form).
Infective endocarditis is a serious condition with mortality rates of up to 76% for EPVE and up to 44% for LPVE. The mortality rate from native valve endocarditis for 442 patients studied from 1945 to 1949 was 44%; currently it is much lower.
In many centers, injection drug users constitute a significant percentage of patients with endocarditis: 7.3% in the Netherlands study. In other areas it is much higher: In three California hospitals between 1965 and 1976, injection drug users accounted for 17%, 43%, and 13% of all cases of Infective endocarditis. The incidence of endocarditis among injection drug users is 2 to 5% per year. The majority of injection drug users with endocarditis have tricuspid valve involvement (32%), the aortic valve is involved in 29%, the mitral valve in 25%, and multiple valves in 9% of cases. Eighty percent of tricuspid valve endocarditis cases in this population are due to Staphylococcus aureus. Nosocomial episodes represented 14.3% of all cases of endocarditis at three hospitals in Ann Arbor, Michigan, between 1976 and 1985.
Pathogenesis
The low incidence of Infective endocarditis is understandable when one considers that infection of the endocardium requires several unrelated factors to be present. Most important, the endocardium must be altered so that the infectious agent can adhere to it; microorganisms must be delivered to this adherent surface; and these pathogens must have surface characteristics that favor their attachment.
Our understanding of the pathogenesis of endocarditis has been greatly enhanced by the use of animal models (rats and rabbits). In animals Infective endocarditis seldom occurs spontaneously, or following intravenous inoculation of microorganisms, unless the endocardial surface has been damaged previously. Placing a polyethylene catheter across the tricuspid valve of rabbits resulted in sterile vegetations on which staphylococcal endocarditis could readily be established. Subsequently, it was shown that endocarditis could be induced by performing the same maneuver on the aortic valve. The minor trauma associated with the placement of the catheter first resulted in the production of sterile vegetations. Histologic examination of vegetations in the rabbit model showed that microorganisms are contained within a densely woven mesh of fibrin with few polymorphonuclear leukocytes within the vegetation. When viridans streptococci were injected intravenously, bacteria were detected histologically in just a few hours, and after 48 hours, 1 mg of vegetation contained as many as 109 bacteria.
Recent studies point to fibronectin — a large glycoprotein that binds avidly to fibrin and platelets — as playing a pivotal role in the adherence of bacteria to the endocardial surface. Immunofluorescent studies have demonstrated that fibronectin is present in the platelet fibrin mass of patients with nonbacterial thrombotic (marantic) endocarditis. Staphylococcus aureus and S. sanguis bind more effectively to fibronectin than do gram-negative bacteria. Thrombospondin is another protein that may promote the adherence of bacteria to the endocardial surface. It is a large (molecular mass 420,000) multifunctional glycoprotein stored in alpha platelet granules. Upon platelet activation, it is released into the fibrin matrix or remains bound to the platelet surface. In turn, bacteria bind to thrombospondin and may contribute to the early stages of development of endocarditis. Other factors, such as complement and protein S (vitronectin), may facilitate the adherence of bacteria to the endothelial cells themselves.
It is believed that the initial process leading to endocardial or subendocardial damage in humans is most often a valvular abnormality, either congenital or acquired, which can lead to endocardial inflammation. This occurs on the mitral, aortic, tricuspid, and pulmonary valves in descending order of frequency. This risk is directly proportional to the pressure gradient existing across these valves. The resulting inflammatory process may mimic the earliest step in the development of nonbacterial thrombotic endocarditis in animals. Infectious endocarditis can develop only when microorganisms are introduced to this surface. Transient bacteremia may develop as a result of normal daily events such as tooth-brushing or from an invasive procedure such as dental extraction or gastrointestinal or genitourinary manipulation. Bacteremia secondary to skin, soft tissue, or vascular catheter infection may also serve as the initiating event, as may direct inoculation at the time of cardiac surgery, which is the cause in early prosthetic valve endocarditis.
Since the number of bacteria that can be cultured from the gingival sulcus approaches 1011/mL of saliva, it is not surprising that activities such as tooth-brushing, chewing hard candy, or the use of oral irrigation devices (such as Waterpik) may result in bacteremia. Viridans streptococci are the most common bacterial species found in the blood in such circumstances. Although anaerobes are found frequently in blood following dental procedures, they cause endocarditis infrequently. Among the viridans streptococci, those that produce dextran polymers on their surface are most likely to produce endocarditis. This is the same material that allows these bacteria to adhere to surfaces in the hostile environment that they find in the oral cavity.
Bacteremia occurs frequently after transurethral prostatic resection (34%), cystoscopy (19%), and urethral catheterization (7%) in the presence of infected urine. Approximately 10% of patients will have bacteremia following sigmoidoscopy or barium enema. Although anaerobic bacteria predominate in the gastrointestinal and genitourinary tracts and may outnumber coliform bacteria and enterococcus species by between 1,000 and 10,000 to 1, enterococci are the most frequent species isolated from blood following manipulation of these areas.
Clinical manifestations
The clinical picture of infective endocarditis is modified by the state of the heart in which the infection occurs (nature of preexisting disease and presence of prosthetic material), age of the patient, and the infecting microorganism. The initial symptoms may suggest a nonspecific, persistent, flulike illness and may include fever, chills, weakness, sweats, anorexia, malaise, or weight loss. Less frequent symptoms, which may be due to the systemic infection or to the vascular reaction, include headache, myalgia, arthralgia, abdominal pain, cough, nausea, vomiting, or edema.
A heart murmur due to underlying cardiac disease is almost invariably present, and the vast majority of patients have a low-grade fever. Skin manifestations are seen in about 25% of patients, the most common being petechiae of the mucous membranes or the skin followed by splinter hemorrhages. Osler’s nodes (red, tender nodules on the pulps of the fingers or toes) or Janeway spots (macular, circumscribed, erythematous lesions on the palms or soles) are less frequent. The spleen is enlarged in about one-quarter of patients, and a minority have clubbing of the digits or retinal lesions (Roth spots — small hemorrhages with a white center). Symptoms may be minimal in older patients infected with less virulent organisms.
New symptoms and signs appear as the illness progresses. Local destruction of a valve results in an acute volume load on the myocardium that may precipitate heart failure and may be associated with a changing murmur. Other factors sometimes contributing to the occurrence of heart failure include coronary emboli, and direct involvement of the myocardium by the infection. Cerebral complications are often devastating. Mycotic aneurysms, due to infected emboli or to vasculitis of the vasovasorum, may rupture and cause massive intracerebral hemorrhage. Large emboli may cause cerebral infarcts, and there is often a relationship between these and the presence of asymptomatic mycotic aneurysms.
Hematuria is frequent and may be due to focal embolic phenomenon or to immune mediated diffuse glomerulonephritis. Septic complications involving the lungs, meninges, joints, brain, and abdominal organs are less frequent The site of the infection within the heart also modifies the manifestation of endocarditis. Right-sided endocarditis due to congenital heart disease, such as ventricular septal defect, tends to be more indolent, with a tendency to pulmonary emboli and less frequent systemic complications. Injection drug users who frequently have endocarditis on the tricuspid valve also tend to have a more indolent course and usually have septic pulmonary emboli.
Certain pathogens, especially fungi, are associated with emboli to large vessels (e.g., the femoral artery). In general, the manifestations are so variable that endocarditis must enter the differential diagnosis of all cases of fever of uncertain origin and must be considered when nonspecific symptoms or signs point to involvement of joints, muscles, lungs, meninges, intra-abdominal organs, kidneys, myocardium, pericardium, or the brain. It also is part of the differential diagnosis of nonspecific illnesses suggesting connective tissue disorders, vasculitis, occult malignancy, or obscure infection.
Diagnosis of endocarditis
In a patient with fever, a systolic ejection murmur and two blood cultures positive for Streptococcus sanguis, the old adage is that this patient has endocarditis until proven otherwise. However, how do you prove it, and what is the degree of certainty that the patient has endocarditis? Durack et al. (1994) proposed a set of criteria that uses pathologic and clinical criteria to place patients with suspected endocarditis into one of three categories — definite, possible, or rejected. Definite endocarditis is said to be present using pathology-based criteria if the microorganism is cultured from a valvular vegetation, or on the basis of typical features of an infected vegetation or cardiac abscess histologically. A diagnosis of definite endocarditis can also be made clinically if there are two major, one major and three minor, or five minor criteria. These diagnostic criteria are shown in Table Major and Minor Criteria for Diagnosis of Infective Endocarditis.
Echocardiography currently plays a key role in the management of patients with endocarditis. The echocardiogram was first used to detect vegetations on the cardiac valves of patients with endocarditis in 1973. Over the years there has been considerable improvement in this technology. Two-dimensional (2-D) transthoracic or transesophageal echocardiography with color Doppler is available in most tertiary-care centers. With transesophageal echocardiography, the imaging probe is close to the cardiac structures, and since there is no interposition of lung or bone, the image quality is superior to that obtained using transthoracic techniques.
| Table Major and Minor Criteria for Diagnosis of Infective Endocarditis | ||
|
The vegetation of bacterial endocarditis varies in size from a few millimeters to greater than 1 cm in diameter. The typical lesion has well-defined borders and a density similar to the myocardium, is located adjacent to a leaflet, but has movement that is partially independent from that of the leaflets themselves. Vegetations can be visualized in 70% of patients with native valve endocarditis by a 2-D transthoracic examination providing that the infection is not on a prosthetic valve. With a transesophageal examination these lesions can be seen in up to 97% of patients with endocarditis, even when the infection is associated with prosthetic valves. Doppler examination is useful in quantifying the degree of valvular regurgitation and the change in this over time. Transesophageal studies can usually demonstrate ring abscesses associated with infection of the aortic valve.
The role of echocardiography in the management of patients with endocarditis has evolved. At one time large vegetations >10 mm in diameter detected by echocardiography implied a poor prognosis and were considered by some experts to be an indication for valve replacement. Now it is evident that the risk of emboli is higher when the vegetation is ≥10 mm in diameter, but size alone is not an indication for valve replacement. All patients with infective endocarditis should undergo baseline transthoracic Doppler echocardiography. Repeat examinations should be limited to patients with important valve or ventricular dysfunction on the initial study, new murmurs, persistent fever, conduction defects, or embolic phenomena. Transesophageal echocardiography is best reserved for patients with prosthetic valve endocarditis, for those with suspected valve ring or intraventricular abscesses, and for patients in whom the transthoracic exam failed to show vegetations (with good clinical evidence of endocarditis) or was technically unsatisfactory. The Duke criteria specify the echocardiographic features that are needed for the diagnosis of endocarditis.
Treatment of endocarditis
The treatment of endocarditis includes the administration of the optimal antimicrobial in the appropriate dosage for the correct duration. However, many other factors should be considered in the management of a patient with endocarditis. A knowledge of the complications of this illness, their investigation, and their treatment is necessary. In addition, patients with endocarditis often have serious preexisting valvular disease, and other co-morbidities that may be worsened by the endocarditis. Since about half of the patients with endocarditis will require cardiac surgery as part of the management of their illness, it is necessary to know the indications for such surgery.
Endocarditis is among the most difficult of infections to treat with antimicrobials. The causative organisms are metabolically inactive, deeply embedded within the vegetation’s matrix, contained within abscess cavities or densely adherent to a prosthetic device, and protected from the host’s natural defense mechanisms. Therapy of bacterial endocarditis therefore requires the prolonged use of bactericidal agents alone or in combination. Removal of prosthetic material and drainage of large abscesses, if present, are often necessary for cure. Combinations of antibiotics may be used with one or more of the following goals in mind:
- To effect a more rapid clinical response
- To shorten the duration of antimicrobial therapy
- To overcome bacterial tolerance
- To change otherwise bacteriostatic agents into a bactericidal combination
- To provide a broader spectrum of antimicrobial coverage during initial empiric therapy
| Table Principles of Management of Endocarditis | |
|
Antimicrobial treatment of bacterial endocarditis most frequently is with a β-lactam antibiotic, either alone or in combination with an aminoglycoside. This combination is usually synergistic, since the b-lactam facilitates the penetration of the aminoglycoside into the bacteria by virtue of its effect on the cell wall (peptidoglycan layer). In patients with endocarditis due to gram-positive bacteria, such as methicillin-resistant S. aureus and coagulase-negative staphylococci, where b-lactams are not effective, vancomycin is usually used. In those circumstances where an aminoglycoside is used in combination with a β-lactam, gentamicin has emerged as the aminoglycoside of choice except for the therapy of endocarditis due to Pseudomonas spp. Enterococci can be highly resistant to gentamicin by virtue of their ability to produce aminoglycoside-inactivating enzymes. Some of these gentamicin-resistant enterococci will be susceptible to streptomycin. Other aminoglycosides such as tobramycin and amikacin are also usually inactive against these highly resistant strains.
Although recommendations relating to the optimal duration of antibiotic therapy for bacterial endocarditis have been published, there is always a need to individualize therapy. A prolonged duration of symptoms before diagnosis, the presence of an abscess or a prosthetic device, unusual or more resistant microorganisms, or the inability to use the optimal antibiotic(s) in recommended dosage may necessitate more prolonged therapy.
Early discharge of patients with continuation of parenteral antibiotics at home has been used for some patients with endocarditis. Although there is little reason to believe that the efficacy of the antibiotics themselves might be reduced complications may rise at home that may require immediate intervention, such as major embolic events, arrhythmias or the sudden onset of congestive heart failure. The risk of such occurrences needs to be balanced with the advantages of early discharge, and candidates for home antibiotics should be carefully selected.
The treatment of right-sided S. aureus endocarditis in injection drug users has undergone changes as a result of recent trials. Three prospective, nonrandomized trials summarized by DiNubile and coworkers (1994) support the use of a 2-week course of a penicillinase-resistant penicillin plus an aminoglycoside to treat uncomplicated right-sided endocarditis caused by methicillin-susceptible S. aureus in this group of patients. Heldman and colleagues (1996) randomized injection drug users with S. aureus right-sided endocarditis to oral therapy with ciprofloxacin 750 mg b.i.d. plus rifampin 300 mg b.i.d for 28 days, or intravenous therapy with oxacillin 2 g every 4 hours or vancomycin 1 g every 12 hours (for penicillin-allergic patients) plus gentamicin for the first 5 days. Forty-four subjects (treatment route: oral 19, intravenous 25) received 28 days of inpatient treatment with the assigned antibiotics. There were four treatment failures, 1 out of 19 in the group receiving oral antibiotics, and 3 out of 25 in the group beginning with 5 days of intravenous antibiotics. Drug toxicity was more common in the intravenous-treated group — largely increases in liver enzymes. This study suggests that for selected patients with right-sided, methicillin-susceptible S. aureus endocarditis, oral ciprofloxacin plus rifampin is effective.
Empiric therapy
Although it is usually recommended that antibiotics be withheld until a microbiologic diagnosis has been made, empiric use of antibiotics may be desirable. In many circumstances it is necessary to make a “best guess” based on the most likely causative organism in the particular clinical setting. General recommendations such as those in Table Suggested Antibiotic Regimens for the Initial Treatment of Suspected Bacterial Endocarditis before the Availability of Blood Culture Results cannot take into account all the factors that might influence the choice and dosage of antibiotics but rather should serve as a guide. Therapy can be modified when culture and susceptibility data are available. Once these data are available, the recommendations of the American Heart Association Council should be followed.
Management of complications
There are many complications that may arise during the treatment of infective endocarditis. These are commonly divided into cardiac and extracardiac (Table Complications of Infective Endocarditis) complications. In one large series only one-fourth of the patients with endocarditis were free of complications
Heart failure
Heart failure complicates the course of 15 to 65% of patients with endocarditis [Add to ref. list or change citation]), and it is the leading cause of death among these patients. If heart failure has resulted from sudden onset of severe valvular insufficiency or obstruction, urgent valve replacement is necessary. Otherwise it can be treated medically, with valve replacement considered for unresponsive cases.
Valve ring abscess/Valve dehiscence
A valve ring abscess complicates 50 to 87% of cases of prosthetic valve endocarditis. These abscesses are much more common with porcine heterografts than with mechanical valves. Since the abscess involves the annulus to which the valve is sutured, valve dehiscence is a common finding. Valve ring abscesses also occur with native valve endocarditis. In one autopsy series, 24 out of 59 (41%) patients with aortic valve endocarditis had a ring abscess (Arnett and Roberts 1976). The following features were predictive of valve ring abscess: infection of the aortic valve; recent valvular regurgitation, pericarditis, high degree of atrioventricular block, short duration of symptoms leading to severe debility or death (Arnett and Roberts 1976).
| Table Suggested Antibiotic Regimens for the Initial Treatment of Suspected Bacterial Endocarditis before the Availability of Blood Culture Results | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Table Complications of Infective Endocarditis | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
Emboli
Emboli are almost a universal complication of endocarditis. At autopsy, 56% of kidneys, 60% of coronary arteries, 44% of spleens, 30% of brains of patients with endocarditis had emboli. Emboli to the central nervous system are the most important clinically; indeed they account for 50% of the neurologic complications of endocarditis. Ninety percent of these emboli lodge in the middle cerebral artery distribution, and recurrent embolization is usually considered an indication for valve replacement. Some workers have refined this indication to recurrence of embolization despite 48 to to 72 hours of appropriate antibiotic therapy, especially if associated with a large vegetation on echocardiography. The incidence rate of emboli is highest with S. aureus endocarditis, with a rate that is 2.4 times than of viridans streptococcal endocarditis. The incidence of emboli is highest during the first week of antimicrobial therapy (13/1000 patient days) falling 10-fold (to 1.2/1000 patient days) after the second week of antimicrobial therapy.
Mycotic aneurysm
This is an uncommon but often devastating complication of Infective endocarditis. Thirty-two patients with mycotic aneurysms were seen at the Mayo Clinic from 1963 to 1979. The aorta, cerebral arteries, visceral arteries, and arteries of the lower and upper extremities are involved in descending order of frequency; 23% of patients have multiple aneurysms. Severe, unrelenting localized headache in a patient with endocarditis suggests the possibility of an intracranial aneurysm. A cranial computerized tomography scan followed by angiography and neurosurgical consultation represents appropriate management.
Multiorgan failure
This complication is not usually identified in reviews of endocarditis. However, it is often the reason for death in patients who have had a complicated course and who have done poorly following cardiac surgery to replace the infected valves. The respiratory, renal, gastrointestinal, and central nervous systems are most commonly involved.
Prolonged fever
Three recent studies have examined the duration of fever in patients with infective endocarditis. Most patients (up to 70%) with endocarditis were afebrile by the end of the first week of therapy. The most common cause for continued fever was infection of the valve ring. Rarely, pulmonary emboli or drug allergy was responsible. Evidence of microvascular phenomena (splinter hemorrhages, mucosal or conjunctival petechiae, Roth spots, Osler’s node, Janeway lesions) and large vessel embolization were associated with prolonged fever in the study by Lederman and colleagues (1992).
Infections due to S. aureus or P. aeruginosa were associated with a mean of 9.1 and 12.3 days of fever (after initiation of antibiotic therapy) respectively, compared with 2.9 days for infections due to enterococci, 3.0 for viridans streptococci, and 3.9 days for coagulase-negative staphylococci. Blumberg and coworkers (1992) performed a case-control study wherein they compared 26 patients with 27 episodes of endocarditis with prolonged fever (temperature of ≥100.4°F for ≥2 weeks) with 26 patients with endocarditis without prolonged fever. Cardiac infection caused prolonged fever in 13 patients, 7 of whom had myocardial abscesses; 16 of the cases required cardiac surgery compared with 2 of the controls (P < 0.001).
Twenty-two of the cases (85%) developed nosocomial complications compared with five of the controls (P < 0.001). An approach to prolonged fever emerges from these three studies. When fever persists for more than 1 week, initiate a thorough investigation. It is necessary to first rule out extension of valvular infection into an adjacent cardiac structure. A transesophageal echocardiogram is the most sensitive investigation for this purpose. Consider metastatic spread of the infection with abscess formation; drug fever; nosocomial infection (the most common cause of which is infection related to the intravenous line) and other underlying illnesses such as malignancy or vasculitis.
Long-term complications
The relapse rate of endocarditis is about 2.7%, and about 4.5% of patients have later recurrent episodes. Late cardiac surgery is required for up to 47% of patients treated medically. Mycotic aneurysms and emboli may occur many months following cure of the endocarditis.
Surgical treatment of endocarditis
From the foregoing it is evident that surgery is frequently necessary for the successful treatment of endocarditis. All patients with prosthetic valve endocarditis should be managed in conjunction with a cardiac surgeon. The indications for urgent cardiac surgery in patients with active endocarditis are given in Table Indications for Urgent Cardiac Surgery in Patients with Active Infective Endocarditis. When indicated, surgery can be performed at any time during the course of endocarditis. For many patients timing of the surgery is most important. This requires experience, good judgment and ongoing cardiological assessment. Unnecessary delay of surgery impairs the outcome. Moderate-to-severe congestive heart failure from valve dysfunction accounts for 90% of the indications for surgery in endocarditis. The availability of human homograft valves has changed the operative approach to endocarditis involving the aortic valve and the aortic root. Porcine and mechanical prostheses still have a place in the management of endocarditis and are usually combined with repairs, using endogenous pericardium and, rarely, prosthetic patch devices.
| Table Indications for Urgent Cardiac Surgery in Patients with Active Infective Endocarditis | |
|
The patient with treated endocarditis and valvular stenosis or insufficiency with intact annular skeletal structures presents little difficulty surgically. The approach is to replace the affected valve or valves with a suitable prosthetic device, and usually, no further surgical intervention is required. These patients have a low incidence of reinfection and do well long- term. The real surgical challenge is the patient with ongoing sepsis who requires urgent intervention for acute heart failure subsequent to valvular destruction and who may have destruction of annular and other contiguous tissue. These patients require aggressive débridement of all infected tissue, which may well involve débridement of part of the ventricular septum, the valvular annulus, the sinus of valsalva, or free wall or septal abscesses. A variety of surgical approaches have been used to effect such débridement and repair.
The principles of surgical treatment of endocarditis are those of surgical treatment of sepsis in general. One must be aggressive in the débridement of all infected and destroyed tissues and be prepared to replace these tissues with suitable substitutes. Once the surgical repair has been completed, the postoperative follow-up of the patient must be done in close cooperation with an infectious disease specialist, as well as a cardiologist. These patients require aggressive continuing antibiotic therapy postoperatively, as well as careful monitoring with regard to further infection and/or valvular deterioration.



