Bacterial Meningitis
Bacterial meningitis remains one of the most feared and dangerous infectious diseases that a physician can encounter. This form of meningitis constitutes a true infectious disease emergency. It is important that the physician quickly make the appropriate diagnosis and initiate antibiotic therapy. Minutes can make the difference between life and death in bacterial meningitis. The rapid progression of disease leaves no time to look through textbooks to decide on appropriate management. To assure the best outcome, every clinician needs a basic understanding of bacterial meningitis and its management.
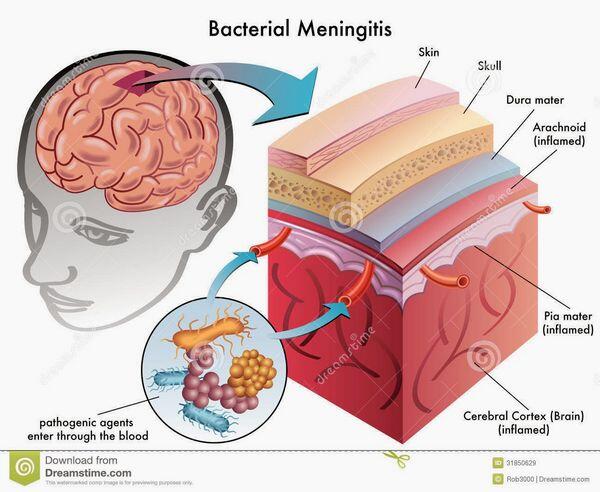
Epidemiology and Causes
With the advent of the Haemophilus influenza В vaccine, the incidence of bacterial meningitis in children declined dramatically in the United States. Bacterial meningitis is now primarily an adult disease. The wider use of pneumococcal vaccine in patients older than 65 years of age and in patients with chronic underlying diseases also promises to reduce the incidence in adults.
Bacterial meningitis is not a reportable disease in the United States, and so an exact incidence is not available, but an estimate places the number at about 3 to 4 per 100,000 population. In underdeveloped countries, the incidence is at least 10 times higher, reflecting crowded conditions, and a lack of vaccination programs as well as other preventive public health measures.
Community-acquired bacterial meningitis in children and adults is caused mainly by four major pathogens:
Streptococcus pneumoniae
S, pneumoniae is the most common cause of community-acquired meningitis in the United States. In other parts of the world, Neisseria meningitidis predominates. S. pneumoniae first causes infection of the ear, sinuses, or lungs, and then spreads to the bloodstream, where it seeds the meninges. S. pneumoniae is also the most common cause of recurrent meningitis in patients with a cerebrospinal fluid leak following head trauma.
Neisseria meningitidis
N. meningitidis can cause isolated, sporadic infection or an epidemic. N. meningitidis first infects the nasopharynx, causing sore throat. In individuals lacking anti-meningococcal antibodies, nasopharyngeal carriage may be followed by bacteremia and seeding of the meninges. Crowded environments, such as college dormitories or military training facilities, increase the risk of N. meningitidis spread. Epidemics usually occur in the winter months when person-to-person transmission by respiratory secretions is most frequent. Patients with defects in terminal complement components are also at increased risk of contracting sporadic meningococcal infection.
Table. Causes of Bacterial Meningitis in Adults
| Community
(%) |
Nosocomial
(%) |
|
| Streptococcus pneumoniae | 38 | 8 |
| Gram-negative bacilli | 4 | 38 |
| Neisseria meningitidis | 14 | 1 |
| Listeria spp. | 11 | 3 |
| Streptococci | 7 | 12 |
| Staphylococcus aureus | 5 | 9 |
| Haemophilus influenzae | 4 | 4 |
Listeria monocytogenes
L. monocytogenes infects primarily individuals with depressed cell-mediated immunity, including pregnant woman, neonates, patients on immunosuppressive drugs, or individuals infected with HIV. People over the age of 60 may also have an increased risk of developing Listeria. This form of meningitis is contracted by ingesting contaminated food. Heavy contamination with Listeria can occur when foods are stored for prolonged periods at 4°C, because the organism can grow in a cool environment. Listeria can contaminate unpasteur-ized soft cheeses and other improperly processed dairy products. High counts of this organism have also been found in defectively processed hot dogs and fish. When Listeria enters the gastrointestinal tract, it is able to silently invade the gastrointestinal lining, enter the bloodstream, and infect the meninges.
Haemophilus influenzae
Before administration of the HIB vaccine became common place, H. influenzae was the most common pathogen to cause meningitis in children; however, meningitis resulting from this organism is now rare.
The causes of bacterial meningitis in neonates reflect the organisms with which they come into contact during passage through the birth canal. Escherichia coli is the most common cause of neonatal meningitis, followed by group В streptococci.
About the Epidemiology and Causes of Bacterial Meningitis
- Primarily a disease of adults.
- Community-acquired disease is associated with four major pathogens:
- Streptococcus pneumoniae is the most common. Meningitis follows bacteremia from ear, sinus, or lung infection. Also associated with chronic leaks of cerebrospinal fluid.
- Neisseria meningitidis begins with colonization of the nasopharynx. Sporadic cases are often associated with terminal complement defects. Epidemics occur in crowded environments such as dormitories and military training camps.
- Listeria monocytogenes occurs in neonates, pregnant women, and immunocompro-mised patients. It is contracted by eating contaminated refrigerated foods.
- Haemophilus influenzae was the most common form of meningitis in children. Following widespread administration of the H. influenzae В vaccine, it is now rare.
- Neonates develop gram-negative and group В streptococcus meningitis.
- Nosocomial meningitis is usually associated with neurosurgery or placement of a ventricu-lostomy tube. It is caused by gram-negative rods, Staphylococcus aureus, enterococci, S. epidermidis, Bacillus subtilis,and corynebacteria.
Nosocomial bacterial meningitis has increased in frequency since the late 1980s. This increased incidence can be explained by the increased numbers of patients undergoing neurosurgical procedures and having hardware placed in the cerebral ventricles. The bacteriology of nosocomial meningitis is very different from that of the community-acquired disease. Gram-negative rods predominate, E. coli and Klebsiella being the most common. Staphylococcus aureus and streptococci are other frequent pathogens. Patients undergoing ventricular shunt placement can develop meningitis from contaminated plastic shunt tubing. S. epidermidis, S. aureus, enterococci, Bacillus subtilis, and corynebacteria (previously called diphtheroids) are most commonly encountered.
Pathogenesis
Bacterial meningitis is most commonly blood-borne. Primary infections of the ears, sinuses, throat, lungs, heart, and gastrointestinal tract can all lead to bacteremia and, on rare occasion, the blood-borne bacteria gain entry into the subarachnoid space. Blood-borne bacteria may gain entry through the large venous sinuses in the brain. Bacteria can settle along these slow-flowing venous channels, then escape and penetrate the dura and arachnoid, infecting the cerebrospinal fluid. Less commonly, bacteria can enter the cerebrospinal fluid through a break in the cribriform plate or a defect in the base of the skull following basilar skull fracture.
Patients with head trauma can develop cerebrospinal fluid leakage at these sites, and bacteria from the nasopharynx or middle ear, primary S. pneumoniae, can track up through the leak into the subarachnoid space. Patients who develop brain abscesses secondary to otitis media and mastoiditis or bacterial sinusitis on rare occasion can develop meningitis because of direct spread of bacteria from the abscess to the subarachnoid space.
About the Pathogenesis of Bacterial Meningitis
- Infectious organisms gain entry to the subarachnoid space and cerebrospinal fluid (cerebrospinal fluid)
- most commonly by bacteremia, gaining entry through the large venous channels;
- by nasopharyngeal spread through a cerebrospinal fluid leak caused by a cribriform plate defect or basilar skull fracture; or
- direct spread from a brain abscess or air sinus infection.
- Rapid growth occurs in the cerebrospinal fluid because the blood-brain barrier blocks entry of immunoglob-ulins and complement.
- Inflammation damages the blood-brain barrier, increasing permeabilityallowing entry of serum protein, and impairing glucose transport.
- Progressive cerebral edema, increased cerebrospinal fluid pressure, and decreased cerebral blood flow lead to irreversible ischemic damage.
Because the blood-brain barrier blocks entry of immunoglobulins and complement, bacteria are able to grow unimpeded by the host’s immune system in the early phases of infection. As the number of organisms increases, polymorphonuclear leukocytes are attracted to the site. As they attempt to kill organisms, polymorphonuclear leukocytes often lyse, releasing toxic oxygen products, proteolytic enzymes, and inflammatory cytokines. These products lead to necrosis and edema of the surrounding tissue.
The marked inflammatory response in the subarachnoid space damages the cerebral microvasculature, increasing the permeability of the blood-brain barrier. Leakage of serum from the damaged vessels increases the protein level in the cerebrospinal fluid.
Inflammation at the surface of the cerebral cortex can induce vasculitis and occlusion of small arteries and cortical veins alike, causing cerebral infarction. Inflammation of the arachnoid and pia matter alters glucose transport into this region, lowering glucose levels in the cerebrospinal fluid. Inflammation of the subarachnoid space may impair cerebrospinal fluid flow and cause hydrocephalus.
Inflammation damages neural cells in the cerebral cortex and causes cerebral edema. The ultimate consequences of intense inflammation and bacterial invasion of the meninges are increased intracranial pressure, decreased cerebral blood flow, and cerebral cortex hypoxia, leading to irreversible ischemic damage.
Case 1
A 47-year-old sales manager and father of two arrived in the emergency room in deep coma. He had a history of recurrent ear infections since age 12. Three days before admission to the hospital,the patient had complained of a severe left earache. He took the ear drops prescribed by his local physician, and the pain disappeared during the night. The evening before he presented to the emergency room, the patient began complaining of headache and feeling “sort of disoriented.” An hour after onset of the headache, he began vomiting, and he vomited five times during the night. The morning of admission, his wife reported that he appeared drowsy. He stayed home from work, sleeping most of the morning. About noon he awoke, but he did not recognize his wife. He began speaking incoherent sentences and became very restless. By 4 p.m., he was failing to respond when his wife called his name, and he was brought to the emergency room.
A physical examination recorded a temperature of 40°C, a blood pressure of 140/100 mm Hg, a pulse of 140 per minute, and a respiratory rate of 20 per minute. This was a very ill-appearing man who did not respond to his name, and who moved all limbs only in response to deep pain.
The patient’s ears were bilaterally blocked with cerumen. The pupils of his eyes were dilated to 8 mm, but reacted to light. Optic disc margins were flat. The neck was very stiff, with both Kernig’s and Brudzinski’s signs present. Coarse diffuse rhonchi were evident throughout all lung fields. No skin lesions were seen. A neurologic exam showed no cranial nerve abnormalities. Reflexes were symmetrical, and the patient moved all limbs.
Laboratory workup found a peripheral white blood cell count of 19,500/mm3, with 39% polymorphonuclear leukocytes (polymorphonuclear leukocytes), 50% band forms, 6% lymphocytes, and 5% monocytes. Hematocrit was 35.5%, and chest X-ray showed no infiltrates.
A lumbar puncture was performed in the emergency room. Opening cerebrospinal fluid pressure was found to be 560 mm H20 (normal: 70 to 180 mm). A cerebrospinal fluid analysis showed a white blood cell count of9500/mm3 (95% polymorphonuclear leukocytes), protein 970 mg/dL (normal: 14 to 45 mg/dL), and glucose 25 mg/dL, with a simultaneous serum glucose level of 210 mg/dL (normal: 50 to 75 mg/dL, generally two thirds of serum glucose). Gram-stain of the cerebrospinal fluid revealed gram-positive lancet-shaped diplococci. Cultures of cerebrospinal fluid and blood grew S. pneumoniae.
Clinical Manifestations of Bacterial Meningitis
Understanding that meningitis is usually the consequence of hematogenous spread from a primary infection, the clinician needs to inquire about antecedent symptoms of ear, nose, and throat infections, as well as about symptoms of pneumonia. The meningitis in case 6.1 was preceded by otitis media.
Case 1 had many of the typical symptoms of meningitis. Classically, patients with bacterial meningitis have symptoms of an upper respiratory tract or ear infection that is abruptly interrupted by worsening fever accompanied by one or more “meningeal” symptoms.
Headache is usually severe and unremitting, often being reported as the most severe headache ever experienced. Generalized pain is the rule, reflecting diffuse inflammation of the meninges. Pain may radiate down the neck. Asparin and other over-the-counter pain medications are usually ineffective.
Neck stiffness is frequently noted and is a consequence of meningeal inflammation precipitating muscle spasms in the back of the neck.
As experienced in case 6.1, vomiting is a frequent symptom. The cause of vomiting is unclear, but may be secondary to brain stem irritation and/or elevated intracerebral pressure.
Altered consciousness usually develops within hours of the onset of headache. As noted in case 1, the patient may become difficult to rouse and often becomes confused and disoriented. Family members often wait surprising long before becoming concerned enough to bring the patient to the hospital. Unfortunately, such delays dramatically worsen the prognosis of bacterial meningitis. In more severe cases, loss of consciousness may be accompanied by grand mal or focal seizures.
Physical examination usually demonstrates high fever or hypothermia. Two maneuvers in addition to a simple test of neck stiffness are commonly used to test for meningeal inflammation:
- Brudzinski’s nape-of-the-neck sign is elicited by flexing the neck forward. This movement stretches the meninges and is resisted by the patient with meningeal inflammation, because the maneuver causes severe pain.
- Kernig’s sign requires that the knee be bent at a 45-degree angle as the patient lies supine. As the leg is straightened, the patient with meningeal irritation will resist straightening, complaining of lower back and hamstring pain.
Although emphasized in most textbooks as key physical findings, neck stiffness and Kernig’s and Brudzinski’s signs have not proved to be sensitive indicators of meningitis. Exacerbation of headache by sudden head movement (head jolt) may be a more sensitive finding.
A careful ear, nose, and throat examination should be performed. Findings of otitis media (dull tympanic membrane, fluid behind the ear drum) may be discovered in cases of S. pneumoniae and H. influenzae or pharyngeal erythema may be noted in cases of N. meningitidis. The nose should be carefully examined looking for a clear nasal discharge suggestive of a cerebrospinal fluid leak. Usually, however, meningeal inflammation temporarily closes the cerebrospinal fluid leak at the time of presentation, such leakage becoming apparent only after the patient recovers. The nasal passage and posterior pharynx may also reveal a purulent discharge suggestive of sinusitis, an infection that less commonly leads to meningitis.
Auscultation of the heart may reveal a diastolic murmur suggesting aortic insufficiency, which would strongly suggest bacterial endocarditis as the primary infection leading to meningitis. Most cases of endocarditis complicated by meningitis are the result of infection with S. aureus.
Lung exam may reveal findings of pneumonia (asymmetrical lung expansion, bronchovesicular breath sounds, rales, egophony, and dullness to percussion), making S. pneumoniae the most likely cause. In all patients with meningitis, a Chest X-Ray should also be performed to exclude pneumonia.
A thorough examination of the skin needs to be performed looking for purpuric lesions. Petechiae and pur-pura are most commonly encountered in patients with meningococcemia, but they may also may be found in S. aureus endocarditis and echovirus 9 and rickettsial infections. In patients who are asplenic, pneumococcal or H. influenzae sepsis is commonly associated with disseminated intravascular coagulation and petechial lesions. The finding of petechiae or purpura is usually a bad prognostic sign.
Finally, and most importantly, a neurologic exam must be performed.
First, mental status must be carefully described. The exact level of neurologic function should be documented by determining a Glasgow score. The level of consciousness on admission is an important criterion for the use of corticosteroids and is also a useful prognostic indicator. The patient who is unresponsive to deep pain (Glasgow score 3) has a much higher mortality than the patient who responds to voice (Glasgow score 10 to 15).
Next, the cranial nerves should be assessed. Lateral-gaze palsy as a result of Vlth nerve dysfunction can result from increased intracranial pressure. Focal findings such as hemiparesis, asymmetric pupillary response to light, or other unilateral cranial nerve deficits are uncommon in bacterial meningitis, and they raise the possibility of a space-occupying lesion such as a brain abscess or tumor. The finding of papilledema on fundo-scopic exam is rare in meningitis and usually indicates the presence of a space-occupying lesion.
Table. Glasgow Coma Score
| E: Eye opening | Spontaneous | 4 |
| Responds to verbal command | 3 | |
| Response to pain | 2 | |
| No eye opening | 1 | |
| V: Best verbal
response |
Oriented | 5 |
| Confused | 4 | |
| Inappropriate words | 3 | |
| Incomprehensible sounds | 2 | |
| No verbal response | 1 | |
| M: Best motor
response |
Obeys commands | 6 |
| Localizing response to pain | 5 | |
| Withdrawal response to pain | 4 | |
| Flexion to pain | 3 | |
| Extension to pain | 2 | |
| No motor response | 1 |
About Clinical Manifestations in Bacterial Meningitis
- Upper respiratory or ear infection interrupted by the abrupt onset of meningeal symptoms:
- Generalized, severe headache
- Neck stiffness
- Vomiting
- Depression of mental status
- Physical findings:
- Brudzinski’s (neck flexion) and Kernig’s (straight leg raise) signs are insensitive;”head jolt” maneuver may have higher sensitivity
- Abnormal ear exam [Streptococcus pneumoniae or Haemophilus influenzae), pharyngeal erythema [Neisseria meningitidis), or clear nasal discharge resulting from a cerebrospinal fluid peak{S.pneumoniae)
- Petechial or purpuric skin lesions most common with N. meningitidis, also seen with rickettsial infection, echovirus 9, Staphylococcus aureus,and asplenic sepsis.
- Neurologic exam should look for focal findings (suggests a space-occupying lesion) and assess mental status (Glasgow score is an important prognostic factor).
It is important to keep in mind that meningitis in very young and very old individuals does not present with these classic symptoms and signs. In elderly people, the onset of meningitis is often more insidious. The earliest symptoms are usually fever and alterations in mental status.
Meningeal signs are less commonly reported, and many elderly patients have neck stiffness as a consequence of osteoarthritis, an old cerebrovascular accident, or Parkinsons disease. The physician must have a high index of suspicion and must aggressively exclude the possibility of bacterial meningitis in an elderly patient with fever and confusion. In very young patients, neonatal and infant meningitis presents simply as fever and irritability.
No history is obtainable, and as a consequence, lumbar puncture should be included in the fever work-up of the very young patient.
Diagnosis
The critical test for making a diagnosis of meningitis is the lumbar puncture. If the clinician has included meningitis as part of the differential diagnosis, a lumbar puncture needs to be performed. Too often, clinicians order a computed tomography scan before performing a lumbar puncture, needlessly delaying the appropriate diagnostic study.
If no focal neurologic deficits are apparent, and if papilledema is not seen on fundoscopic examination, a lumbar puncture can be safely performed. The major exception is patients with AIDS or those receiving immunosuppressants. These patients have a higher frequency of cortical space-occupying lesions.
At the time of lumbar puncture, cerebrospinal fluid pressure should be documented by manometry. In cases of bacterial meningitis, cerebrospinal fluid pressure is almost always elevated, and high elevation suggests severe cerebral edema or defective cerebrospinal fluid resorption, or both.
Cellular and biochemical analysis of the cerebrospinal fluid is very helpful in deciding on the most likely cause of meningitis.
Patients with bacterial meningitis who have not received prior antibiotics have increased numbers of White blood cells with more than 90% polymorphonuclear leukocytes in their cerebrospinal fluid. Patients with L. monocytogenes can have a lower percentage of polymorphonuclear leukocytes. Listeria grows and survives within the cytoplasm of host cells, a condition that can stimulate a monocytic cerebrospinal fluid response in some patients. Patients who have received antibiotic therapy before their lumbar puncture may also have a reduced percentage of polymorphonuclear leukocytes.
Because bacterial meningitis causes marked inflammation of the meninges, glucose transport is impaired, and cerebrospinal fluid glucose is usually low (“hypoglycorrachia”). Normally, the cerebrospinal fluid glucose concentration is about two thirds that of serum glucose; a blood sample for serum glucose should therefore be drawn at the time of the
Table. Profiles of Cerebrospinal Fluid in Central Nervous System Infections
| Type of infection | White blood cell type | Glucose | Protein |
| Untreated bacterial | Polymorphonuclear leukocytes | Low
(often <25 mg/dL) |
Elevated (150-1000 mg/dL) |
| Tuberculous, fungal, treated bacterial | Lymphocytes | Low | Moderately elevated (80-500 mg/dL) |
| Viral | Lymphocytes | Normal
(low in early mumps) |
Moderately elevated (usually <150 mg/dL) |
| Parameningeal (brain abscess) | Polymorphonuclear leukocytes or lymphocytes | Normal | Normal or slightly elevated |
Lumbar puncture to more accurately assess the cerebrospinal fluid glucose level. In patients with pneumococcal meningitis, a cerebrospinal fluid glucose level below 25 mg/dL is associated with worse clinical outcome.
As a consequence of inflammatory damage to blood vessels within the meninges, serum leaks into the cerebrospinal fluid, causing a rise in protein concentration.
About the Diagnosis of Bacterial Meningitis
- If meningitis is a consideration, a lumbar puncture must be performed.
- If focal neurologic deficits and papilledema are absebt, a lumbar puncture can be performed before computed tomography scan.
- Opening pressure of the cerebrospinal fluid (cerebrospinal fluid) should be measured; it is often elevated.
- The cerebrospinal fluid formula is very helpful in deciding whether a patient has bacterial meningitis. Bacterial meningitis is suggested in the presence of
- more than 90% polymorphonuclear leukocytes (except with Listeria),
- elevated cerebrospinal fluid protein (usually 150 to 1000 mg/ dL),and
- low cerebrospinal fluid glucose [less than two thirds the serum value (less than 25 mg/dL is prognostic of poor outcome)].
- Gram stain of cerebrospinal fluid is positive in more than 75% of cases (except 25% with Listeria).
- Blood and cerebrospinal fluid cultures allow for antibiotic sensitivity testing.
Concentrations can reach 1000 mg/dL in some cases, and the cerebrospinal fluid protein almost always exceeds the normal adult concentration of 50 mg/dL in cases of bacterial meningitis.
The combination of polymorphonuclear leukocytes, low glucose, and high protein in the cerebrospinal fluid is almost always caused by bacterial meningitis, and the finding of this cerebrospinal fluid formula warrants treatment with antibiotics.
In addition to cerebrospinal fluid cell, glucose, and protein levels, Gram stain and culture of the cerebrospinal fluid are needed. In more than 75% of bacterial meningitis cases, the Gram stain is positive. The exception is cases involving L. monocytogenes. Because this organism usually remains intracellular, Gram stain is positive in only 25% of cases. Latex agglutination tests for H. influenzae, S. pneumoniae, and N. meningitidis are available, and may be ordered in patients with a negative Gram stain.
However, it must be emphasized that the sensitivity of these tests is somewhat variable, and a negative latex agglutination test does not exclude the possibility of bacterial meningitis.
The cerebrospinal fluid culture should be planted immediately after lumbar puncture, and in the absence of prior antibiotics, it remains the most sensitive test for diagnosis. In addition, a positive culture allows for antibiotic sensitivity testing, which is particularly important for guiding treatment of S. pneumoniae and enteric pathogens.
Treatment
Evaluation and institution of antibiotic therapy should occur within 30 minutes if bacterial meningitis is being strongly considered. In cases in which a focal neurologic deficit is evident or papilledema is found, empiric antibiotic therapy should be instituted before sending the patient for computed tomography scan. Blood samples for culture should be drawn before antibiotics are started; they often yield the cause of the illness. Empiric antibiotic treatment is also required if Gram stain of the cerebrospinal fluid proves negative.
Empiric therapy depends on the age and immune status of the patient and on whether infection is nosocomial or community-acquired. For community-acquired meningitis in patients aged 3 months to 60 years, maximal doses of a third-generation cephalosporin (ceftriaxone or cefotaxime) is recommended. If the patient is severely ill, vancomycin should be added to this regimen to cover for the possibility of penicillin-resistant S. pneumoniae.
In the patient with an immediate hypersensitivity reaction to penicillin or a history of allergy to cephalosporins, vancomycin is recommended. In patients over the age of 60 years, maximal doses of ampicillin are added to the third-generation cephalosporin to cover for L. monocyto-genes.
his organism is not sensitive to cephalosporins, and penicillin or ampicillin are the treatment of choice. For the immunocompromised host, a third-generation cephalosporin, ampicillin, and vancomycin are recommended for empiric therapy. In patients post neurosurgery or in patients who have a cerebrospinal fluid shunt, vancomycin and ceftazidime or cefepime are recommended.
Once a specific bacterium is identified, the antibiotic regimen can be focused.
Penicillin-resistant S. pneumoniae is a particular concern, given the high prevalence of these strains and the poor penetration of antibiotics across the blood-brain barrier. Intermediately resistant stains (penicillin minimum inhibitory concentration = 0.1 to 1 µg/mL) may initially improve on penicillin therapy; however, as the integrity of the blood-brain barrier improves, the patient may relapse as a consequence of reduced levels of penicillin in the cerebrospinal fluid. For this reason, high-dose ceftriaxone or cefotaxime is recommended for intermediately penicillin-resistant S. pneumoniae meningitis, because these cephalosporins achieve higher levels in cerebrospinal fluid.
Table. Antibiotic Treatment for Bacterial Meningitis
| Organism | Antibiotic | Dose | Alternative |
| Streptococcus pneumoniae (penicillin minimum inhibitory concentration < 0.1 (ig/mL) | Penicillin
Ceftriaxone Cefotaxime |
20-24 X 106 U daily, divided q4h 2-4 g daily, divided q12h 12 g daily, divided q6h | Chloramphenicol: 4-6 g daily, divided q6h |
| Streptococcus pneumoniae (minimum inhibitory concentration > 0.1 (xg/mL) | Vancomycin, plus rifampin 300 mg q12h | 2g daily, divided q12h | Chloramphenicol |
| Neisseria meningitidis | Penicillin | 20-24 X 106 U daily, divided q4h | Ceftriaxone Cefotaxime |
| Listeria monocytogenes | Ampicillin, with or without gentamicin | 12 g daily, divided q4H 4-8 mg intrathecal 5 mg/kg daily systemic | Trimethoprim-sulfamethoxazole 15-20 mg/kg daily (trimethoprim, divided q6h) |
| Haemophilus influenzae | Ceftriaxone Cefotaxime | 2-4 g daily, divided q12h 12 g daily, divided q6h | Chloramphenicol |
| Enterobacteriaceae | Ceftriaxone, with or without gentamicin | 2-4 g daily, divided q12h 4-8 mg intrathecal | Aztreonam 6-8 g daily, divided q6h |
| Cefotaxime, with or without gentamicin | 5 mg/kg daily, systemic 12 g daily, divided q6h | Trimethoprim-sulfamethoxazole | |
| Pseudomonas aeruginosa | Ceftazidime, plus gentamicin | 6-12 g daily, divided q8h 4-8 mg intrathecal 5 mg/kg daily systemic | Antipseudomonal penicillin: 18-24 g daily, divided q4h, plus gentamicin |
| Staphylococcus aureus (methicillin-sensitive) | Nafcillin, or
Oxacillin,with or without rifampin |
9-12 g daily, divided q4h 9-12 g daily, divided q4h 600 mg daily,divided q12h | Vancomycin, plus rifampin Trimethoprim-sulfamethoxazole, plus rifampin |
| S. aureus (methicillin-resistant) | Vancomycin, plus rifampin | 2g daily, divided q12h 600 mg daily, divided q12h | |
| S. epidermidis | Vancomycin, plus rifampin | 2g daily, divided q12h 600 mg daily,divided q12h |
For infections with highly penicillin-resistant S. pneumoniae (penicillin minimum inhibitory concentration >2 µg/mL), vancomycin needs to be added to the third-generation cephalosporin to assure adequate inhibitory concentrations in the cerebrospinal fluid. Vancomycin penetrates the intact blood-brain barrier poorly, and in some patients, therapeutic levels may not be achieved in the cerebrospinal fluid without intrathecal administration. Rifampin combined with vancomycin may also be effective for the treatment of highly resistant S. pneumoniae.
This regimen has been recommended for patients receiving high-dose dexamethasone (see discussion of treatment for inflammation that follows), because corticosteroid therapy reduces meningeal inflammation and improves the integrity of the blood-brain barrier, decreasing vancomycin levels in the cerebrospinal fluid. The antibiotic response should be monitored in patients infected with highly penicillin-resistant pneu-mococci. In these patients, the lumbar puncture should be repeated 24 to 36 hours after the initiation of therapy.
Aminoglycosides, erythromycin, clindamycin, tetracyclines, and first-generation cephalosporins should not be used to treat meningitis, because these drugs do not cross the blood-brain barrier.
Neurologic damage is primarily a consequence of an excessive inflammatory response. Corticosteroids reduce inflammation, and in children with H. influenzae bacterial meningitis, dexamethasone (0.15 mg/kg q6h X 4 days) has been shown to reduce cerebrospinal fluid pressure, cerebrospinal fluid polymorphonuclear leukocytes, and protein, to increase cerebrospinal fluid glucose, and to improve cerebral blood perfusion. Dexamethasone also significantly reduces the incidence of deafness. In adults with pneumococcal meningitis and Glasgow coma scores of 8 to 11, dexamethasone administration (10 mg q6h X 4 days) was also found to reduce morbidity and mortality.
Dexamethasone should be given just before or simultaneously with antibiotics, because inflammatory mediators are released in response to the lysis of bacteria induced by antibiotic treatment. Other inhibitors of inflammation, such as monoclonal antibodies directed against the adherence receptors of leukocytes, are potentially promising, but remain experimental.
Additional therapeutic measures are primarily directed at reducing cerebral edema and controlling seizures. Administration of hypotonic solutions should be avoided. The airway must be protected, and hypoventilation with associated hypercarbia should be avoided, because elevated PaCO2 levels cause cerebral vessel dilation and may increase intracranial pressure. Hyperventilation can also be harmful for the opposite reason: reductions in PaC2 may reduce cerebral perfusion and increase the risk of infarction.
When intracranial pressure is documented by lumbar puncture to be markedly elevated, intravenous 20% mannitol can be administered to remove free water from the cerebral cortex and to quickly reduce cerebral edema. Oral glycerol may also reduce cerebral edema, and its efficacy is presently being investigated. Seizures develop in 20% to 30% of patients with meningitis, but anti-seizure medications (Dilantin and diazepam are most commonly used) are not recommended for prophylaxis. These agents are administered only after the first seizure.
Complications
Mortality remains high in patients with bacterial meningitis. L. monocytogenes is associated with the highest mortality, 26%; followed by S. pneumoniae, 19%; and N. meningitidis, 13% mortality. H. influenzae meningitis tends to be less severe, being now associated with an average mortality of 3%. Mortality is higher in very young and elderly individuals. Neurologic sequelae in surviving patients are common. The young patient whose brain is developing often suffers mental retardation, hearing loss, seizure disorders, or cerebral palsy. Older patients may develop hydrocephalus, cerebellar dysfunction, paresis, a seizure disorder, and hearing loss.
About the Treatment of Bacterial Meningitis
- Antibiotics should be given within 30 minutes if bacterial meningitis is suspected.
- Blood samples for culture should be drawn and antibiotics given before a computed tomography scan is done.
- Maximal doses of antibiotics must given because of limited passage through the blood-brain barrier.
- Empiric therapy for
- community-acquired disease, patient 3 months to 60 years is ceftriaxone or cefotaxime. If severely ill, add vancomycin. If more than 60 years or immunocompro-mised, use ceftriaxone or cefotaxime, plus ampicillin and vancomycin.
- nosocomial disease, is vancomycin and ceftazidime or cefepime.
- Give dexamethasone 30 minutes before antibiotics in
- children (shown to be efficacious in Haemo-philus influenzae).
- adults (efficacious in Streptococcus pneumoniae with Glasgow coma score of 8 to 11).
- Maintain ventilation, prevent increase in PaCO2 or decrease in PaO2.
- Avoid hypotonic solutions, consider mannitol or glycerol for increased cerebrospinal fluid pressure.
- Anti-seizure medications after first seizure.
Prevention
Given the high mortality and high incidence of permanent neurologic sequelae associated with bacterial meningitis, the medical community must strive to reduce the incidence of these devastating infections.
Vaccines
Three of the primary pathogens that cause community-acquired bacterial meningitis are encapsulated organisms, and therefore opsonins [immunoglobulin G and complement] play a critical role in allowing host macrophages and polymorphonuclear leukocytes to ingest these pathogens and clear them from the bloodstream. Reduced time in the bloodstream reduces the likelihood of seeding the meninges. The remarkable reduction in invasive H. influenzae type В following the widespread administration of the HIB vaccine illustrates the power of this preventive measure. Protective levels of immunoglobulin are achieved when the PedvaxHIB vaccine is administered at 2 and 4 months of age. Two other HIB vaccines are also available that should be administered at 2, 4, and 6 months of age.
A quadrivalent meningococcal vaccine directed against serogroups A, C, Y, and W135 is now available and is recommended for high-risk groups, including military recruits, college students, asplenic patients, and patients with terminal complement deficiencies. This vaccine is also useful for controlling epidemics and should be administered to travelers going to areas where the prevalence of meningococcal disease is high, (Visit www.CDC.gov for current recommendations for travelers.)
A major problem with the current vaccine is the lack of a suitable immunogen against serogroup B. Serogroups В and С are primarily responsible for meningococcal meningitis in the United States. A second problem with the vaccine is the fact that immunity tends to be short-lived, with antibody titers decreasing after 3 years following a single dose of the vaccine. The incidence of meningococcal disease remains low in the United States (approximately 1 in 100,000 population), and therefore this vaccine is not recommended for routine immunization.
A safe, inexpensive, and efficacious 23-valent pneumococcal vaccine is available and has been underutilized.
The mortality attributable to pneumococcal infection is higher than that attributable to any other vaccine-preventable disease (approximately 40,000 annually in the United States), and about half of these deaths could be prevented by vaccination. Individuals more than 65 years of age are at higher risk for developing invasive pneumococcal infection including meningitis and should be vaccinated.
Other groups that warrant vaccination include patients with chronic cardiovascular, pulmonary, or liver disease, diabetes mellitus, and sickle cell disease, and patients with functional asplenia or those who have had a splenectomy A single intramuscular or subcutaneous injection is protective for 5 to 10 years.
For most patients, revaccination is not recommended. Exceptions are the immunocompro-mised host and patients over 65 years of age who often develop a more rapid decline in protective antibody levels. Revaccination may considered after at least 5 years have passed since initial vaccination. A heptavalent conjugated vaccine that is immunogenic in children under the age of 2 years is recommended for routine pediatric immunization. This vaccine has significantly reduced invasive pneumococcal disease in children. It is given in four doses at 12 to 15 months, and ages 2, 4, and 6.
About the Outcome and Prevention of Bacterial Meningitis
- Mortality is high: 26% for Listeria, 19% for Streptococcus pneumoniae, 13% for Neisseria meningitidis, and 3% for Haemophilus influenzae.
- Permanent sequelae are common:
- In children: mental retardation, hearing loss, seizure disorders, cerebral palsy
- In adults: hydrocephalus, cerebellar dysfunction, paresis, seizure disorder, hearing loss
- Efficacious vaccines are available:
- S. pneumoniae: 23-valent vaccine; safe, inexpensive. Recommended in individuals more than 65 years of age; those with chronic cardiovascular, pulmonary, or liver disease, diabetes mellitus, sickle cell disease, and asplenia; heptavalent conjugated vaccine for all children under 2 years of age.
- H. influenzae: PedvaxHIB vaccine at age 2 and 4 months; safe, inexpensive.
- N. meningitidis: quadrivalent meningococcal vaccine for serogroups A, C, Y, and W135; misses group B. Recommended in military recruits, college students, and individuals with asplenia and terminal complement defects.
- Chemoprophylaxis use:
- H. influenzae: Rifampin within 6 days for household contacts with unvaccinated child under 2 years of age, and for children under 2 years of age exposed in a daycare center.
- N. meningitidis: Single-dose ciprofloxacin within 5 days for household and daycare contacts,and for those exposed to oral secretions from the index case.
Chemoprophylaxis
Brief antibiotic treatment has been used to prevent secondary cases of H. influenzae and N. meningitidis. Secondary cases generally occur within 6 days of an index case of H. influenzae and within 5 days of an index case of TV meningitidis meningitis. Both organisms are carried in the nasopharynx and, in a person lacking specific humoral immunity, these organisms can become invasive.
Choice of the individuals to target for prophylaxis has been carefully delineated by epidemiologic data, but fear plays a major role in determining who eventually receives prophylaxis. For H. influenzae, household contacts with at least one unvaccinated child under the age of 2 years require prophylaxis. Data on daycare exposure remains controversial; however, most experts agree that children under the age of 2 who may have been exposed in a day-care should receive chemoprophylaxis.
The recommended agent for H. influenzae prophylaxis is rifampin 20 mg/kg daily (maximum dose in adults: 600 mg q24h) for 4 days is recommended. Rifampin prophylaxis is not recommended for pregnant woman because of the potential risk of rifampin to the fetus. For N. meningitidis, a single dose of ciprofloxacin 500 mg is the preferred prophylactic regimen, and this regimenis recommended for close contacts including household members, daycare contacts, and people who may have been direcdy exposed to the index patients oral secretions (kissing, mouth-to-mouth resuscitation, endotracheal tube intubation).
Given the potential severity of this disease and the minimal harm of a single dose of antibiotic, physicians should probably maintain a low threshold for using prophylaxis. This brief treatment may help to alleviate the extreme anxiety associated with meningococcal disease.
Viral Meningitis
Case 2
A 45-year-old woman was admitted to the hospital with a chief complaints of severe headache and neck stiffness over 8 days. Ten days prior to admission, she had noted some mild stiffness of the back of her neck,associated with fever and mild shivering. Two days later, she developed a sharp, throbbing bi-temporal headache that radiated to the vertex. Her headache was made worse by sitting up or moving. Bright light bothered her eyes. She also noted some muscle stiffness in other areas in particular her lower back. She felt very tired and lost her appetite. Although she felt lethargic at times, she never lost touch with reality.
An epidemiologic history revealed that during the fall (several weeks before admission), she had administered psychometric tests to a large number of students (ages 10 to 20 years).
Physical examination found a temperature of 38°C. This mildly ill-appearing middle-aged woman was alert but sitting in a dark room complaining of severe headache. Eyes showed mild conjunctival erythema with normal discs. Neck was mildly stiff and negative forKernig’s and Brudzinski’s signs. The remainder of the exam, including ear, nose, and throat and neurologic exams, was within normal limits.
Laboratory workup showed a hematocrit of 40%; a white blood cell count of 6000/mm3, with 45% polymorphonuclear leukocytes, 50% lymphocytes, and 5% monocytes. Lumbar puncture showed an opening pressure of 100mm H2O,and cerebrospinal fluid analysis found a white blood cell count of 180/mm3 (50% polymorphonuclear leukocytes, 48% lymphocytes, 2% monocytes), protein 59 mg/dL, and glucose 61 mg/dL, with simultaneous serum glucose 84 mg/dL. A Gram stain of the cerebrospinal fluid was negative for organisms. A repeat lumbar puncture 8 hours later revealed an opening pressure of 100 mm H2O, and a white blood cell count of 170/mm3 (2%> polymorphonuclear leukocytes, 95% lymphocytes, 3% monocytes), protein 58 mg/dL, and glucose 61 mg/dL. (No blood for serum glucose was drawn at this time.) Gram stain of the cerebrospinal fluid remained negative.
During this patient’s hospital course, her headache persisted, as did her low-grade fever. She remained alert and continued to have photophobia and a mildly stiff neck. She was discharged on the third hospital day, and her symptoms resolved over the next week.
Viral meningitis is the most common form of meningitis. It is caused primarily by the non-polio enteroviruses, echoviruses, and coxsackieviruses. In temperate climates, infections occur mainly in the warmer months of the year, usually during the summer and early fall. In tropical climates, the infection occurs year round.
Enteroviruses are spread by the fecal-oral route, and small epidemics are frequently reported. Herpes simplex virus type 2 (HSV-2) is the second most common cause, and this form of viral meningitis is often accompanied by vesicular skin lesions in the genital area. This virus is also the most common cause of recurrent Mollaret’s aseptic meningitis. Varicella virus is the third most common cause, and aseptic meningitis usually is not accompanied by skin lesions.
In the nonimmune patient, mumps virus is often associated with aseptic meningitis that may occur in the absence of salivary gland swelling. The peak incidence of this virus is seen in children 5 to 9 years of age. Less commonly, herpes simplex virus type 1 (HSV-1) causes meningitis. And the mononucleosis syndromes caused by Epstein-Barr virus and cytomegalovirus can be accompanied by meningitis.
Lymphocytic choriomeningitis virus was previously thought to be a common cause of aseptic meningitis, but recent studies have found this virus to be rare. It is transmitted in the urine of rodents, and a diagnosis of lymphocytic choriomeningitis should be considered in individuals who potentially have had contact with rodents or rodent excreta. This infection occurs most commonly in the winter, when rodents are more likely to take up residence in human dwellings.
Finally, at the time of initial HIV infection, 5% to 10% of patients may experience symptoms of aseptic meningitis. In some of these cases, HIV has been isolated from the cerebrospinal fluid.
Severe headache is the most common complaint. Headache is usually generalized, but may localize bilaterally to the frontal, temporal, or occipital regions. Photophobia is another very common complaint, and patients usually request that their room remain darkened. Neck stiffness and diffuse myalgias are also common.
On physical examination, the skin should be carefully viewed for maculopapular rashes (found in some strains of echovirus). Eye exam may reveal conjunctivitis, frequently associated with enteroviral infections. Significant nuchal rigidity is found in more than half of all cases of aseptic meningitis. Patients may be slightly lethargic; however, unlike patients with bacterial and fungal meningitis, patients with viral meningitis rarely exhibit significant depression in mental status. Focal neurologic findings should not be observed in this disease.
Lumbar puncture usually reveals a predominance of lymphocytes, a normal glucose level, and mildly elevated cerebrospinal fluid protein. The cerebrospinal fluid leukocyte count usually ranges between 100 and 1000 /mm3. In some forms of viral meningitis (mumps and lymphocytic choriomeningitis), cerebrospinal fluid glucose may be lowered early in the disease. Also early in the disease, polymorphonuclear leukocytes may predominate in the cerebrospinal fluid, making it impossible to safely exclude bacterial meningitis.
These patients should therefore not be sent home, but covered with empiric antibiotics pending cerebrospinal fluid and blood cultures and follow-up lumbar puncture. In most cases, a repeat lumbar puncture 12 to 24 hours later reveals a predominance of lymphocytes, and the patient can be discharged.
However, in some patients, polymorphonuclear leukocytes may persist for up to 48 hours, necessitating continued observation in the hospital and antibiotic administration. A negative cerebrospinal fluid culture after 48 hours gready reduces the probability of bacterial meningitis, but the threshold for antibiotic coverage must be low to prevent inadvertent delays in the treatment of a bacterial meningitis.
Polymerase chain reaction for HSV-1 and HSV-2 in cerebrospinal fluid is sensitive and specific, and available in most hospital laboratories. Enterovirus Polymerase chain reaction has also been shown to be sensitive and specific, but the test is not usually available in hospitals. Proof of enterovirus cerebrospinal fluid infection would allow the patient to discharged home, because, with the exception of patients with severe immunoglobulin deficiency, viral meningitis is a self-limiting disease that usually resolves spontaneously within 7 to 10 days. In patients with agammaglobulinemia,a chronic enteroviral meningitis (“meningoenchephalitis”) can develop that continues for years. This condition is often fatal. Treatment with systemic and intraventricular pooled Immunoglobulin G preparations has been successful in some of these patients.
About Viral Meningitis
- Viral meningitis is most commonly caused by
- enteroviruses, echovirus, and coxsackievirus (most frequent, seen in summer and early fall).
- mumps in the nonimmune (may be no parotid gland swelling,ages5to9years).
- herpes simplex type 2 (primary disease, also Mollaret’s recurrent meningitis).
- Epstein-Barr virus and cytomegalovirus (rare).
- lymphocytic choriomeningitis virus (excreted in rodent urine, rare).
- HIV (can be the initial presentation of infection).
- Primary clinical manifestations include
- headache and photophobia, stiff neck;
- no loss of consciousness; and
- conjunctivitis,maculopapular rash,and occasionally with echovirus, petechial rash.
- Epstein-Barr virus and cytomegalovirus (rare).
- The cerebrospinal fluid (cerebrospinal fluid) shows a predominance of lymphocytes, early polymorphonu-clear leukocytes (polymorphonuclear leukocytes), normal glucose, mild protein increase.
- Polymerase chain reaction can make the diagnosis of HSV-1 or -2 and enterovirus, but diagnosis is often presumptive.
- Treatment consists mainly of observation, with antibiotics if cerebrospinal fluid contains polymorphonuclear leukocytes; self-limiting disease, lasts 7 to 10 days.
Tuberculous Meningitis
Tuberculous meningitis arises most commonly in association with miliary tuberculosis. Meningitis can also develop if a tubercle ruptures into the subarachnoid space. About 25% of patients have no evidence of an extracranial site of tuberculous infection.
The symptoms and signs of tuberculous meningitis vary. In some patients, it can mimic other forms of acute bacterial meningitis; in others, the disease is more indolent and presents with a mild headache and malaise. Because tuberculous meningitis involves primarily the basilar meninges, inflammation often involves the pons and optic chiasm, leading to dysfunction of the Illrd, IVth, and VIth cranial nerves, causing abnormalities in extraocular movements and the pupillary response. Changes in mental status need to be carefully documented; outcome correlates closely with the neurologic findings. Patients who are stu-porous or have hemiplegia have a nearly 50% risk of dying or suffering severe neurologic sequelae.
About Tuberculous Meningitis
- Usually develops during miliary tuberculosis.
- No pulmonary disease is evident in 25% of cases.
- Clinically similar to other forms of meningitis:
- Basilar process involving the pons and optic chiasm
- Deficitsofthe Illrd,IVth,andVlth cranial nerves
- Non-communicating hydrocephalusa possibility
- Development of coma a bad prognostic sign
- The cerebrospinal fluid (cerebrospinal fluid) obeys the “500 rule”: fewer than 500 white blood cells, usually lymphocytes; protein less than 500 mg/dL; glucose often less than 45 mg/dL.
- Culture should use large volumes of cerebrospinal fluid; smear for acid-fast bacilli is positive in one third of cases; polymerase chain reaction is a sensitive test.
- Fatal if not treated within 5 to 8 weeks. Treatment with isoniazid, rifampin, and pyrazi-namide;add corticosteroids for hydrocephalus.
In most children, but in only 50% of adults, a Chest X-Ray demonstrates changes consistent with tuberculosis. A purified protein derivative test is helpful and is usually positive. However, a negative purified protein derivative does not exclude the diagnosis.
Lumbar puncture is the key to diagnosis, usually obeying the “500 rule.” That is, the leukocyte count is usually below 500/mm3 (usual range: 100 to 500/mm3), and protein is usually below 500 mg/dL (range: 100 to 500 mg/dL). In addition, a moderate depression in cerebrospinal fluid glucose is usually encountered (below 45 mg/dL); however, in a significant number of cases, cerebrospinal fluid glucose may exceed this value.
A predominance of mononuclear leukocytes is the usual cellular response; however, early in tuberculous meningitis, polymorphonuclear leukocytes may predominate in up to one quarter of patients. A cerebrospinal fluid smear for acid-fast bacilli is positive in slightly more than one third of cases, but repeat examination of multiple samples that have been centrifuged increases the sensitivity. Large volumes of cerebrospinal fluid should be collected for culture to increase the culture yield. Amplification tests using Polymerase chain reaction for tuberculosis are now available. They are highly specific, but their sensitivity does not match culture.
A negative cerebrospinal fluid Polymerase chain reaction therefore does not exclude the diagnosis. A computed tomography or magnetic resonance imaging scan with contrast may reveal rounded densities indicative of tuberculomas, basilar arachnoid inflammation, and hydrocephalus. Flow of cerebrospinal fluid may be impaired as a consequence of basilar inflammation that blocks travel through the aqueduct of Sylvius.
After appropriate cultures are obtained, treatment should be initiated immediately. Untreated tuberculous meningitis is fatal within 5 to 8 weeks of the onset of symptoms. Prognosis is worse in patients under the age of 5 year or over the age of 50 years. A three-drug regimen consisting of isoniazid, rifampin, and pyrazinamide is recommended.
Ethambutol or streptomycin can be added if infection with a resistant organism is suspected. In addition to antituberculous agents, a glucocorticoid (adults: 60 mg prednisone daily; children: 2 to 4 mg/kg daily) or dexamethasone (adults: 10 mg intravenously every 6 hours; children: 0.4 mg/kg daily given intravenously every 6 hours) is recommended in patients with hydrocephalus so as to reduce basilar inflammation.
Cryptococcal Meningoencephalitis
Cryptococcus neoformans is found predominantly in pigeon droppings. High concentrations of this yeastlike fungus are found in pigeon nesting areas and on ledges where pigeons perch. The organism is inhaled and subsequently gains entry into the bloodstream, where it seeds the brain and meninges, causing a meningoencephalitis.
Cryptococcus has a thick capsule consisting of negatively charged polysaccharides that are immunosup pressive, blocking both cell-mediated immune responses and leukocyte migration.
About Cryptococcal Meningoencephalitis
- Transmitted by pigeon excreta.
- Inhaled, infects the lung, bloodstream, meninges, and brain.
- Yeast, with a thick capsule that is immunosup-pressive. Produces melanin and mannitol.
- Symptoms wax and wane, and diagnosis often delayed for more than 1 month.
- Headache is the most common symptom.
- Personality change and confusion develop as disease progresses.
- Stiff neck is uncommon.
- Deficits of the lllrd, IVth,Vlth,and Vlllth cranial nerves can occur.
- A lumbar puncture is required for diagnosis; increased cerebrospinal fluid pressure often associated.
- White blood cells 20 to 200/mm3, with a predominance of mononuclear cells
- Mildly elevated protein and moderately depressed glucose
- Positive India ink preparation in 25% to 50% of cases,and positive cryptococcal antigen in approximately 90%
- Culture usually positive in 5 to 7 days
- Computed tomography or magnetic resonance imaging scan with contrast may show hydro-cephalus, cerebral edema, and ring-enhancing lesions (cryptococcomas).
- Treat with amphotericin В and flucytosine for 2 weeks, fluconazole for 3 to б months.
- Mortality is 25% to 30%; prognosis is worse if cerebrospinal fluid produces a positive India ink preparation, an antigen titer higher than 1:32, a white blood cell count below 20/mm3, or increased opening pressure; or if extraneural infection is present.
These effects explain the minimal inflammatory response elicited by invading cryptococci. Strains that produce melanin demonstrate increased virulence, and this cell wall product is thought to provide protection against oxidants. The high concentrations of dopamine in the Central nervous system serve as a substrate for melanin production. Cryptococcus also produces mannitol, a product that may induce cerebral edema and inhibit phagocyte function.
Cryptococci infect immunocompromised hosts most commonly, but infections in normal hosts are also reported. This form of meningitis is the most common in patients with AIDS. In the non-HIV-infected patient, cryptococcal Central nervous system infection usually has a slowly progressive, waxing and waning course, characterized by severe intermittent headache, followed by mild confusion and personality changes that can progress to stupor and coma. The subacute onset and nonspecific nature of this illness often delay the diagnosis. On average, the diagnosis is determined 1 month after the onset of symptoms. The progression of this illness tends to be more rapid in HIV-infected patients, and the larger burden of organisms results in marked inhibition of the inflammatory response.
Like Mycobacterium tuberculosis, Cryptococcus produces a basilar meningitis that can cause oculomotor palsies because of dysfunction in the lllrd, IVth, Vlth cranial nerves, hearing loss, and hydrocephalus. Patients may experience decreased visual acuity and diplopia. Neck stiffness is often minimal, and the possibility of meningoencephalitis may not be considered. Papilledema is noted in up to one third of cases. Focal motor deficits and seizures are rare.
The diagnosis is made by lumbar puncture. Pressure of the cerebrospinal fluid is often elevated above 200 mm H20, reflecting disturbances in cerebrospinal fluid flow and resorption. The cerebrospinal fluid formula typically has 20 to 200 White blood cells/mm3, with a predominance of mononuclear cells, mildly elevated protein, and moderately decreased glucose.
The cerebrospinal fluid can be mixed one-to-one with India ink, and this preparation reveals encapsulated rounded particles in 25% to 50% of infected patients without HIV. Lymphocytes and starch granules can be mistaken for yeast forms. True cryptococcal forms have a double refractile wall, a distinctly outlined capsule, and refrac-tile inclusions within their cytoplasm. The most useful finding is a budding yeast form, which, when encountered, provides strong proof of a true cryptococcal infection.
Cryptococcal polysaccharide antigen latex agglutination is highly sensitive and specific. The cerebrospinal fluid antigen titer is determined by serially diluting the cerebrospinal fluid. In most cases of HIV-associated cryptococcal meningitis, cryptococcal antigen can also be detected in the serum.
However, a negative serum antigen test does not exclude cryptococcal meningitis in the normal host. A cerebrospinal fluid culture is positive in 90% of patients, and cultur-ing large volumes of CSF (10 to 15 mL) can increase the yield. The organism usually grows within 5 to 7 days on standard media, and use of birdseed agar can enhance growth. In addition to cerebrospinal fluid analysis, brain computed tomography or magnetic resonance imaging scan with contrast is recommended to assess the degree of hydrocephalus and the extent of cerebral edema, and to look for the presence of discrete, ring-enhancing masses called cryptococcomas.
In the patient who does not have AIDS, the goal of therapy is to eradicate the infection. Conventional amphotericin В (0.5 to 0.7 mg/kg daily) or lipid preparations (5 mg/kg daily) and flucytosine (100 to 150 mg/kg daily given in four divided doses) are recommended for a minimum of 2 weeks. If the patient has improved clinically, therapy can be switched to oral fluconazole (400 mg daily), with consolidation therapy continued for 3 to 6 months. In patients who respond poorly to therapy, a lumbar puncture should be repeated at 2 weeks. Amphotericin В and flucytosine should be continued until cerebrospinal fluid cultures are sterile.
Mortality for cryptococcal meningitis is 25% to 30% in patients who do not have AIDS. Poor prognostic factors include a positive cerebrospinal fluid India ink preparation, a cerebrospinal fluid cryptococcal antigen titer in excess of 1:32, a cerebrospinal fluid white blood cell count below 20/mm3, elevated cerebrospinal fluid opening pressure, and extraneural infection.


