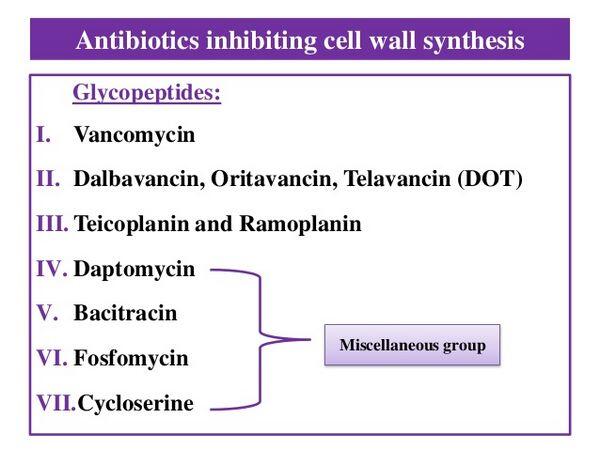
Overview
Glycopeptides are used for the treatment of severe or life-threatening infections that are caused by gram-positive organisms such as streptococci and staphylococci. In acute exacerbations of chronic bronchitis , they are used rarely. Physicians typically reserve them for severe acute exacerbations of chronic bronchitis episodes in which multidrug-resistant organisms are suspected or in which the patient has complications, such as bacteremia or meningitis. They are also used as second-line agents in patients who cannot receive or who have failed to respond to penicillins and cephalosporins.
Glycopeptide therapy is relatively well tolerated. The most severe adverse reactions are toxicities associated with the inner ear and kidneys, but they occur only infrequently. Hypersensitivity, of which skin rash is the most common allergic reaction, is reported in 5-10% of patients. Other possible side effects of glycopeptides include GI disturbances, muscle pain, blood disorders (e.g., eosinophilia), and disturbances in liver enzymes.
Mechanism Of Action
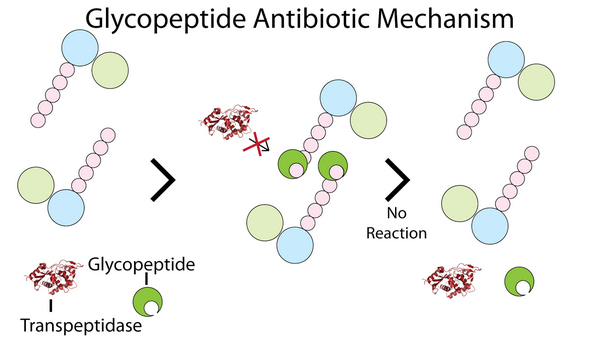
Glycopeptides inhibit bacterial cell-wall biosynthesis by blocking glycopeptide polymerization. This effect produces immediate inhibition of cell-wall synthesis and secondary damage to the cytoplasmic membrane. Glycopeptides also alter the permeability of the cytoplasmic membrane and directly inhibit RNA synthesis. However, the impact these effects have on bacterial viability is largely unknown. These agents have a concentration-independent bactericidal action resulting primarily from inhibition of cell-wall synthesis; they are considered slowly bactericidal against staphylococci.
Vancomycin
Vancomycin (Lilly’s Vancocin, generics) is a narrow-spectrum, bactericidal glycopeptide antibiotic. Vancomycin was developed in the 1950s and has been available worldwide since the 1960s; it is available in oral and IV formulations.
Because this agent has been available for an extensive period of time, no high-quality clinical studies pertaining to acute exacerbations of chronic bronchitis have been conducted in recent years. Thus, this section does not cite specific efficacy results from clinical studies for vancomycin.
Physicians use the injectable glycopeptides extensively to treat systemic infections because these compounds are active against gram-positive bacteria such as S. pneumoniae. Vancomycin has a relatively long duration of action and therefore can be administered every 12 hours.
Toxicity to the inner ear has occurred in patients receiving vancomycin and can be transient or permanent. Most cases have been reported in patients receiving high IV doses or in those with a history of hearing complications. Vancomycin is also associated with kidney toxicity, and patients are screened for a history of renal complications. Renal failure has been reported in patients receiving high doses of IV vancomycin, and physicians exercise particular caution when treating patients concomitantly with another aminoglycoside. Vancomycin can also cause infusion-related flushing (“red man syndrome”) in some patients.
Resistance to vancomycin can occur through a modification of the components of the bacterial cell wall. Vancomycin binds to a component of the cell wall that when modified or altered results in reduced binding of the drug to the bacteria. However, no cross-resistance between vancomycin and other antibacterial agents has been observed in gram-positive microbes.
Teicoplanin
Teicoplanin (Sanofi-Aventis’ Targocid) is a narrow-spectrum, bactericidal glycopeptide antibiotic similar in structure to vancomycin. It was first launched in France and Italy in 1988 and by 1997 was available in most of Europe. It was launched in Japan in 1997, where Aventis has partnered with Fujisawa for distribution and co-promotion, but has never been available in the United States, where development was suspended in 1999.
Teicoplanin is available in parenteral formulations and has a significantly longer duration of action than vancomycin, thus allowing once-daily administration. Unlike vancomycin, teicoplanin can be given by IM as well as by IV injection.
Considered a valuable alternative to vancomycin, teicoplanin is used as empirical therapy in immunocompromised patients with hematological malignancies.
The effectiveness of teicoplanin has been documented in several indications including lower respiratory infections. An open multicenter Phase III trial in Europe to evaluate the efficacy of teicoplanin enrolled a total of 1,431 hospitalized patients. Most patients had S. aureus infections, 19.6% of which were methicillin-resistant. Teicoplanin monotherapy was used in 1,037 cases and in combination with other antibacterials in 394 cases. An overall favorable clinical success was seen in 91.7% of the 1,333 evaluable cases.
The most common adverse reactions shared with glycopeptides generally include inner ear and kidney toxicities. Teicoplanin has fewer propensities than does vancomycin to cause toxicities such as inner ear and kidney toxicity, or infusion-related flushing (“red man syndrome”). Hypersensitivity reactions are also a concern of physicians when considering this therapy.


