Essentials of Diagnosis
- Enteritis caused by Shigella species may be watery (Shigella sonnei, Shigella boydii) or dysenteric (Shigella dysenteriae, Shigella flexneri).
- Risks include ingestion of fecally contaminated food or water and contact with infected individuals.
- Definitive diagnosis requires microbiologic isolation and identification of Shigella species or molecular evidence of infection.
General Considerations
Epidemiology
Dysentery is a disease of antiquity and has been described throughout the ages. It was not until the 19th century that dysentery was recognized to be caused by either parasitic amoebae or certain bacteria. In 1898, Shiga recognized and isolated bacteria from patients with dysentery that would agglutinate when exposed to the patient’s serum. Today, the most commonly recognized agents of bacterial dysentery are Shigella species and the EIEC (see above).
Shigella species are unique among bacterial enteric pathogens in that < 200 and possibly = 10 organisms may transverse the gastric acid barrier and cause disease. For this reason, person-to-person transmission is common. Person-to-person transmission results in increased frequencies of shigellosis in day care centers, schools, and custodial-care facilities. Disease is most common in infants and young children and frequently occurs in family members of patients. Peak incidence occurs in the summertime, and common houseflies are thought to contribute to the spread of disease. Outbreaks have also occurred from fecally contaminated food. Transmission through contaminated water is most common in developing countries that lack adequate sewage and water treatment facilities.
In the United States, S sonnei is the most commonly encountered Shigella species, whereas S boydii has a worldwide distribution. The prevalence of shigellae appears to be cyclic, with replacement of the predominant strain approximately every 20 years. This cycling of prevalence is presumably secondary to slowly acquired herd immunity in a given host population. Epidemic shigellosis, caused by S dysenteriae and S flexneri, is prevalent in underdeveloped countries, but may develop anywhere that poverty, overcrowding, or conditions of war exist.
Microbiology
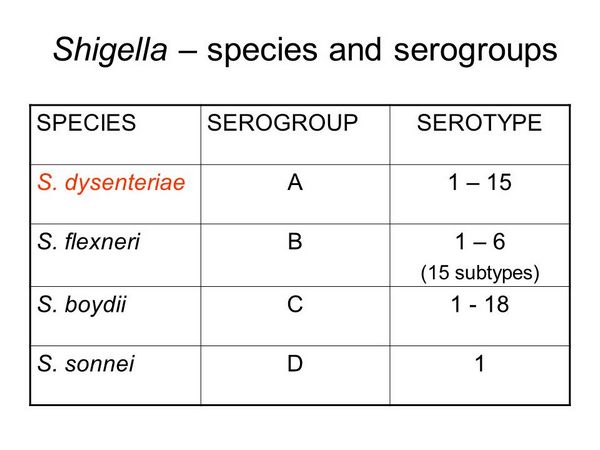
Shigella are nonmotile, facultative anaerobic, gram-negative bacilli that are closely related to the genus Escherichia. At least 40 serotypes compose four groups or species. These are S dysenteriae (serogroup A), S flexneri (serogroup B), S boydii (serogroup C), and S sonnei (serogroup D).
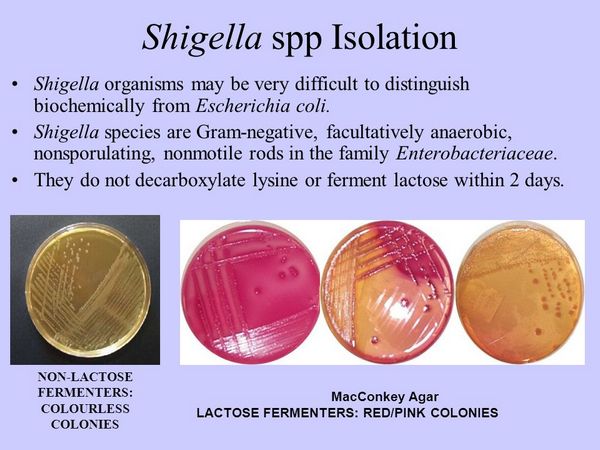
The numbers of shigellae present in the stool vary with the course of disease. Early in the watery-diarrhea phase, shigellae are abundant and number 103-109 shigellae/g of feces. During this phase of disease, shigellae are easily recovered on MacConkey or eosin methylene blue (EMB) agar, where they appear as lactose nonfermenting colonies. Later in the course of disease, in the dysentery and postconvalescent phases, bacterial stool counts decline to 102-103 shigellae/g of feces. Furthermore, the recovery of shigellae is inversely proportional to specimen transport time, especially in stool specimens with a low number of shigellae. During the latter phase of disease, culture is best accomplished by rapid specimen transport or bedside medium inoculation, combined with the use of enrichment broth and moderately to highly selective media, such as xylose-lysine-desoxycholate medium and Shigella-Salmonella medium.
In many laboratories, suspect colonies, lactose nonfermenters, are screened by using a three-tube set: (i) one tube containing triple sugar iron (TSI) or Kligler iron agar (KIA), (ii) the second containing lysine iron agar (LIA), and (iii) the third containing Christensen’s urea agar (CU) (also see the Salmonella Microbiology section below). On the TSI and KIA, shigellae characteristically produce an alkaline slant and acid butt without the production of gas. Rare isolates may produce gas. Negative reactions are produced on the LIA and CU, because shigellae do not decarboxylate lysine or hydrolyze urea. In addition, shigellae do not produce hydrogen sulfide, which is detected by the TSI, KIA, and LIA systems. An attempt to agglutinate organisms that are thought to represent Shigella species may be performed by using group antisera. Isolates with a suggestive screen profile are further characterized by additional biochemical reactions in either traditional or automated systems.
Useful clues in the identification of shigellae include the following: the majority of shigellae cannot ferment mucate, cannot use acetate, and are negative for indole and ortho-nitrophenyl-ß-galactopyranoside.
Pathogenesis
Shigellosis may produce either predominantly watery diarrhea or watery diarrhea that progresses to dysentery. The severity of disease is largely determined by the invading organism. S dysenteriae and S flexneri are the agents most commonly associated with bacillary dysentery, whereas the other Shigella species more often produce watery diarrhea.
The pathogenesis of the watery-diarrhea phase of bacillary dysentery is caused by a combination of lumenal bacterial replication and superficial mucosal invasion in the small intestine. During this phase of disease, large numbers of shigellae are present in the lumen of the small intestine. This phase of the disease is correlated with the onset of cramping abdominal pain, fever, and toxemia.
Within days, the lumenal contents of the small intestine do not contain shigellae, and the site of infection is the colon. The shigellae invade colonic mucosa and occasionally invade to the level of the submucosa. Factors that are important for invasion are present on the bacterial chromosome, as well as on a 140-MDa plasmid. Eventually, epithelial cell death occurs, and the mucosa sloughs, possibly secondarily to shigatoxin production. The loss of mucosa evokes an intense inflammatory response and allows for the introduction of coliform bacteria. Microabscesses, epithelial ulcerations, and pseudomembranes that consist of sloughed epithelial cells, bacteria, fibrin, and inflammatory cells may be seen. This phase of the disease correlates with tenesmus and fractionated stools that contain blood, mucus, and inflammatory debris.
Clinical Findings
Signs and Symptoms
Early in the course of disease, when bacteria are present in the small intestine, patients develop acute, watery diarrhea; fever; and abdominal pain. Patients may become toxemic and fever may reach as high as 104 °F. Later in the course of disease, the primary site of infection is the colon. In this phase, fever continues, but is usually less pronounced. The pain that is present is usually in the lower abdominal quadrants. Stools become dysenteric, consisting of a mixture of neutrophils, blood, mucus, and debris. Frequent, small-volume or fractionated stools may occur, and tenesmus is often present. Patients experience pain upon rectal examination. Colonoscopy discloses hyperemic and friable-to-ulcerated colonic mucosa.
Differential Diagnosis
When watery diarrhea predominates, other bacterial, parasitic, and viral enteric pathogens must be considered. Entamoeba histolytica and EIEC must also be considered in patients with dysentery. Infection with E histolytica is most commonly associated with travel to or living in endemic locales. These organisms are readily recognized on a microscopic examination of the stool for ova and parasites. Dysentery caused by the EIEC, however, may pose a diagnostic challenge for the clinical microbiologist. The EIEC, unlike other E coli, are often nonmotile, may not ferment lactose or may ferment it slowly, are lysine decarboxylase negative, and may cross-react with Shigella antisera. Laboratory personnel must recognize this potential pitfall and exclude this organism through additional testing (see above).
Noninfectious causes of diarrhea must also be considered. The differential diagnosis of noninfectious colitis is extensive and includes inflammatory-bowel disease, lymphocytic/collagenous colitis, neoplasia, and numerous other disorders. Patients with inflammatory-bowel disease may also have fecal leukocytes, limiting the usefulness of this test. An accurate diagnosis may be achieved through a thorough history and physical examination, excluding enteric pathogens through appropriate microbiologic studies, and by obtaining and reviewing gastrointestinal biopsies via endoscopy and histopathologic studies.
Complications
The most common complications of bacillary dysentery include dehydration and a protein-losing enteropathy. In rare instances, a toxic megacolon may occur and may result in perforation, intra-abdominal hemorrhage, peritonitis, and possibly death. Some patients, particularly those with HLA-B27 phenotypes, may develop a postinfectious arthritis or Reiter’s syndrome.
Diagnosis
Patients with acute diarrhea, which may be watery to dysenteric; fever; abdominal pain; and systemic symptomatology/toxemia may have shigellosis. A history of exposure to individuals with shigellosis, travel to endemic areas, and exposure to a high-risk population, such as persons in a custodial-care facility, should raise the index of suspicion. The presence of leukocytes in the stool, although supportive, is by no means definitive for shigellosis. Fecal leukocytes may be present in the stools of patients with other bacterial enteritides, amoebic dysentery, pseudomembranous colitis, and noninfectious disease, such as inflammatory-bowel disease. The definitive diagnosis requires the microbiologic identification of a Shigella species.
Shigellae are particularly susceptible to some environmental changes, and they die rapidly in transport. Therefore, it is imperative to rapidly transport the stool of patients suspected of having shigellosis to the laboratory. This is especially important for patients in the latter stages of disease, in whom the number of shigellae in the stool are relatively few.
Treatment
Fluid and electrolyte replacements are necessary for patients with dehydration. In most instances, this is readily accomplished by oral rehydration. Unlike in many other bacterial enteritides, antibiotic therapy is important in the treatment of shigellosis (Box 4). Antibiotic therapy limits the clinical course of the disease, may decrease the likelihood of intestinal complications, and decreases the fecal excretion of viable pathogenic organisms, which in turn diminishes transmission. Fluoroquinolones are the treatment of choice for adults. TMP/SMX is the treatment of choice for children. Alternatives are ampicillin, chloramphenicol, and nalidixic acid. In areas of known resistance to TMP/SMX, such as parts of Southeast Asia, Africa, and South America, quinolones should be used for adults, and one of the above mentioned alternatives for children with shigellosis. When available, the antimicrobial-susceptibility profile should guide therapy.
Antimotility agents, such as diphenoxylate, should not be used. The inhibition of diarrhea increases the contact between the intestinal mucosa and the pathogenic organisms and their toxins and may cause more fulminant disease.
Prognosis
The prognosis is generally good for patients with endemic or sporadic shigellosis. Infants and the elderly, especially if malnourished, suffer the highest mortality. Epidemic shigellosis caused by S dysenteriae, however, is a severe and often life-threatening disease with mortality rates from 5% to 20%. This disease must be treated aggressively with antimicrobial and rehydration therapies.
Prevention & Control
The development and refinement of sewage disposal and drinking water treatment systems are important in developing countries. In both developed and developing countries, personal hygiene, good hand washing practices, and clean living conditions are important preventive measures, particularly in custodial-care facilities (see Box 3). Fly control and hygienic food preparation practices should also diminish the incidence of disease.





