Essentials of Diagnosis
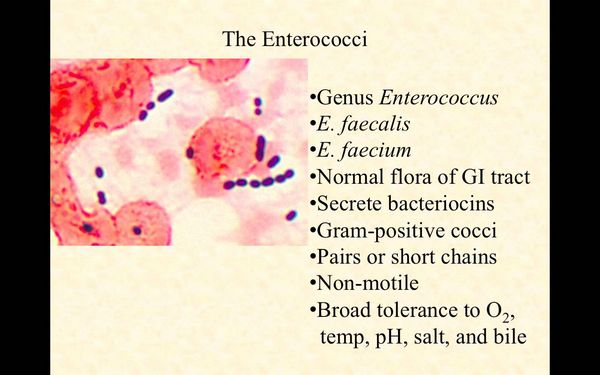
- Gram stain shows gram-positive cocci that occur in singles, pairs, and short chains; recovery of microorganism from culture of blood or other sterile source.
- Lancefield group D antigen.
- Clinical isolates: Enterococcus faecalis, 74%; E faecium, 16%; other species, 10%.
- Facultative anaerobes grow in 6.5% NaCl at pH 9.6 and at temperatures ranging from 10 °C to 45 °C, and grow in the presence of 40% bile salts and hydrolyze esculin and L-pyrrolidonyl-ß-naphthylamide.
- Infections typically of a gastrointestinal or genitourinary origin.
- The most common infections are urinary tract infection, bacteremia, endocarditis, intra-abdominal and pelvic infection, and wound and soft tissue infection.
General Considerations
Epidemiology
Enterococci are able to grow and survive under harsh conditions and can be found in soil, food, water, and a wide variety of animals. The major habitat of these organisms is the gastrointestinal tract of humans and other animals, where they make up a significant portion of the normal gut flora. Most enterococci isolated from human stools are E faecalis, although E faecium are also commonly found in the human gastrointestinal tract. Small numbers of enterococci are occasionally found in oropharyngeal and vaginal secretions and on the skin, especially in the perineal area. Because enterococci are part of the normal gut flora of almost all humans, infections caused by these organisms may be endogenously acquired from the patient’s own flora.
Enterococcal infections also occur in hospitalized patients or in patients undergoing peritoneal or hemodialysis, and the organisms causing such infections often appear to be exogenously acquired. There is clear-cut evidence for spread of strains of enterococci between patients and even for dissemination of such strains from one institution to another. Strains of enterococci causing nosocomial infections have been found on the hands of medical personnel and on environmental surfaces in hospitals and in nursing homes. Most likely, enterococci from patients or hospital personnel first colonize the gastrointestinal tract (or occasionally the skin and groin or other contiguous areas) before causing infections in other patients. Devices such as electronic rectal thermometers may also aid in the spread of enterococci, especially antibiotic-resistant enterococci.
Enterococci account for 12% of nosocomial bacterial infections. The most common nosocomial enterococcal infections involve the urinary tract (62% of cases) and are often associated with urinary tract instrumentation or structural abnormalities. Wound infections are the next most common presentation of nosocomial enterococcal infections, usually involving abdominal or pelvic sites (25% of cases). Bacteremia (10% of cases) is the third most commonly reported nosocomial enterococcal infection.
Risk factors for acquiring nosocomial enterococcal infection or colonization, especially those caused by vancomycin-resistant enterococci (VRE), include being critically ill; having severe underlying disease; being immunosuppressed (especially patients on oncology or transplant wards); having renal insufficiency; undergoing intra-abdominal or cardiothoracic surgical or other invasive procedures; having an indwelling urinary or central venous catheter; having a prolonged hospital or intensive care unit stay; intrahospital transfer between floors; multi-antimicrobial agent, third-generation cephalosporin, anti-anaerobic antimicrobial or vancomycin therapy; proximity to infected patients; care by colonized staff; and the receipt of selective bowel decontamination, sucralfate, or enteral feedings.
Microbiology
Enterococci are gram-positive cocci that occur in singles, pairs, and short chains. The genus Enterococcus contains over a dozen species. E faecalis isolates account for ~74% of organisms encountered in the clinical microbiology laboratory. E faecium accounts for 16% of such isolates. The other Enterococcus species, including E durans, E avium, E casseliflavus, E gallinarum, E raffinosus, and E hirae (among others), are encountered clinically in ~10% of cases. Enterococci are facultative anaerobes that are able to grow in 6.5% NaCl at pH 9.6 and at temperatures ranging from 10 to 45 °C. They carry the Lancefield group D antigen and will grow in the presence of 40% bile salts. They hydrolyze esculin and L-pyrrolidonyl-ß-naphthylamide.
Enterococci are characterized by intrinsic resistance to many antibiotics (eg, oxacillin, clindamycin, cephalosporins, and aminoglycosides) and the capacity to acquire resistance to many others. The uniformly poor activity of ß-lactams, particularly cephalosporins, against enterococci results from reduced affinity of the penicillin-binding proteins. Unlike most streptococci, enterococci are inhibited but not killed by apparently active penicillins (ampicillin, mezlocillin, penicillin, piperacillin), carbapenems (imipenem, meropenem), and glycopeptides (vancomycin or teicoplanin). Cephalosporins are not clinically useful against enterococci.
Enterococci are also naturally resistant to clinically achievable levels of aminoglycosides used alone. For treating serious enterococcal infections, combination therapy is recommended, generally penicillin, ampicillin, or vancomycin with an aminoglycoside, usually gentamicin or streptomycin. The rationale for this approach is to weaken the cell wall with the cell wall-active agent, thereby facilitating entry of the then bactericidal aminoglycoside. Such combinations have been shown to be synergistic, as long as the organism is susceptible to the cell wall-active agent and does not demonstrate high-level resistance to the aminoglycoside (gentamicin minimum inhibitory concentration = 500 ug/mL, streptomycin minimum inhibitory concentration = 2000 ug/mL).
Acquired resistance in enterococci can develop by genetic mutation or by acquiring altered DNA from a resistant organism. Enterococci have been reported to have acquired high-level resistance to aminoglycosides, cell wall-active agents (including penicillins and vancomycin), chloramphenicol, clindamycin, erythromycin, and the newer quinolones. E faecium isolates exhibit more antibiotic resistance than do other species.
Most enterococci with high-level resistance to aminoglycosides contain aminoglycoside-modifying enzymes. When high-level resistance to aminoglycosides is a concern, only gentamicin and streptomycin minimum inhibitory concentrations need to be tested in the clinical microbiology laboratory because the enzyme that neutralizes gentamicin also modifies tobramycin, amikacin, kanamycin, and netilmicin but not streptomycin. In addition to enzymatic resistance, ribosomal modification has been described as a second mechanism for high-level streptomycin resistance.
Acquired resistance to ampicillin and penicillin is generally caused by altered penicillin-binding proteins. This confers higher levels of resistance than the intrinsic resistance present in enterococci. This mechanism confers resistance to all ß-lactams (including the carbapenems). ß-lactamase production among enterococci is rare and has been reported in a few hospitals.
In the background of increasing resistance of enterococci to penicillins and aminoglycosides, vancomycin resistance emerged in the late 1980s. In vancomycin-resistant cells, an abnormal pentapeptide peptidoglycan precursor, with which vancomycin interacts minimally, is synthesized and incorporated into the cell wall of the organism. Resistance to the glycopeptide antibiotic vancomycin in enterococci, as understood to date, is phenotypically and genotypically heterogeneous. Three glycopeptide resistance phenotypes, VanA, VanB, and VanC, have been described in enterococci; they can be distinguished by the level and inducibility of resistance to vancomycin and teicoplanin. VanA-type glycopeptide resistance is characterized by acquired inducible resistance to both vancomycin and teicoplanin, and it is transferable. VanB-type glycopeptide resistance is characterized by acquired inducible resistance to various concentrations of vancomycin but not to teicoplanin, and it is also transferable. VanC-type glycopeptide resistance is characterized by low-level vancomycin resistance but teicoplanin susceptibility and has been described as an intrinsic property of most isolates of E gallinarum, E casseliflavus, and E flavescens. VanD-, VanE- and VanG- type glycopeptide resistance have been recently described and do not appear to be common.
Pathogenesis
Little is known about specific pathogenesis and virulence factors of enterococci. Enterococcal bacteremia has, however, been associated with high mortality rates (42-68%). In many cases, enterococci cause infections in severely debilitated hosts and are part of a polymicrobial infection. Thus their independent contribution to mortality and morbidity is difficult to assess. The intrinsic and acquired resistance of enterococci to many antimicrobial agents, as discussed previously, is an important factor that allows these organisms to survive and proliferate in patients receiving antimicrobial therapy. In addition, enterococci are able to adhere to heart valves and renal epithelial cells, properties that likely contribute to their ability to cause endocarditis and urinary tract infections, respectively.
Although enterococci are found in one-fifth of intra-abdominal infections, their exact role in polymicrobial infection is controversial. In cases of intra-abdominal infections, selective therapy against Escherichia coli and Bacteroides fragilis, which has minimal in vitro activity against enterococci, has been found to be sufficient to reduce enterococcal counts. Nevertheless, in animal models of experimental polymicrobial intra-abdominal infection, enterococci have been found to enhance abscess formation, weight loss, and mortality. Similarly, clinical reports have indicated the emergence of enterococcal abscesses and bacteremia after treatment of intra-abdominal sepsis with antimicrobial agents that lack significant in vitro enterococcal activity. A recent multicenter study of intra-abdominal infection has found that the presence of enterococci in the initial cultures, in addition to serious underlying disease, independently predicts treatment failure with broad-spectrum antimicrobial regimens that lack specific enterococcal activity.
Enterococci: Clinical Syndromes
Table 1. Recommendations for preventing the spread of vancomycin resistance: prudent vancomycin use.
Situations in Which the Use of Vancomycin Is Acceptable
Discouraged
- For treatment of serious infections caused by ß-lactam-resistant gram-positive microorganisms
- For treatment of infections caused by gram-positive microorganisms in patients who have serious allergies to ß-lactam antimicrobial agents
- When antibiotic-associated colitis fails to respond to metronidazole therapy or is severe and potentially life-threatening
- For prophylaxis as recommended by the American Heart
- Association for endocarditis following certain procedures in patients at high risk for endocarditis (see site)
- Prophylaxis for major surgical procedures involving implantation of prosthetic materials or devices (eg, cardiac and vascular procedures and total hip replacement) at institutions that have a high rate of infections caused by methicillin-resistant Staphylococcus aureus or methicillin-resistant S epidermidis. (A single dose of vancomycin administered immediately before surgery is sufficient unless the procedure lasts more than 6 h, in which case the dose should be repeated. Prophylaxis should be discontinued after a maximum of two doses)
- Routine prophylaxis other than in a patient who has a life-threatening allergy to ß-lactam antibiotics
- Empiric antimicrobial therapy for a febrile, neutropenic patient unless initial evidence indicates that the patient has an infection caused by gram-positive microorganisms (eg, an inflamed exit site of a Hickman catheter) and the prevalence of infections caused by methicillin-resistant S. aureus in the hosipital is substantial
- Treatment in response to a single blood culture positive for coagulase-negative Staphylococcus spp. if other blood cultures taken during the same time frame are negative (ie, if contamination of the blood culture is likely) (Because contamination of blood cultures with skin flora (eg, S epidermidis) could result in inappropriate administration of vancomycin, phlebotomists and other personnel who obtain blood cultures should be trained to minimize microbial contamination of specimens)
- Continued empiric use for presumed infections in patients whose cultures are negative for ß-lactam-resistant, gram-positive microorganisms
- Systemic or local (eg, “antibiotic lock”) prophylaxis for infection or colonization of indwelling central or peripheral intravascular catheters
- Selective decontamination of the digestive tract
- Eradication of methicillin-resistant S aureus colonization
- Primary treatment of antibiotic-associated colitis
- Routine prophylaxis of very-low-birth-weight infants (ie, infants who weigh less than 1,500 g)
- Routine prophylaxis for patients on continuous ambulatory peritoneal dialysis
- Treatment chosen for dosing convenience of infections caused by ß-lactam sensitive, gram-positive microorganisms in patients who have renal failure
- Use of vancomycin solution for topical application or irrigation
BOX 1. Enterococcal Infections
More Common
- Urinary tract infection
- Bacteremia
- Endocarditis
- Wound and soft tissue infection
- Intra-abdominal and pelvic infection
Less Common
- Meningitis
- Neonatal sepsis
BOX 2. Treatment of Enterococcal Infections
Uncomplicated Urinary Tract Infection
Bacteremia, Intraabdominal or Pelvic Infection,Wound or Soft Tissue Infection, and Neonatal Sepsis
First Choice
- Amoxicillin, 250-500 mg orally every 8h
OR
- Ampicillin, 250-500 mg orally every 6 h
- Aqueous crystalline penicillin G, 18-30 million U/24 h IV either continuously or in 6 equally divided doses with or without gentamicin2 (1 mg/kg IV/IM every 8h)
OR
- Amipicillin sodium 12 g/24 h IV in 6 equally divided doses with or without gentamicin2 (1 mg/kg IM/IV every 8 h)
Second Choice
- Nitrofurantoin, 50-100 mg orally every 6 h
OR
- Ofloxacin, 200 mg orally every 12 h
OR
- Ciprofloxacin, 250-500 mg orally every 12 h
- Levofloxacin, 250 mg orally every d
OR
- Norfloxacin, 400 mg orally every 12 h
OR
- Enoxacin, 200-400 mg orally every 12 h
OR
- Gatifloxacin, 200-400 mg orally every d
- Vancomycin, 30 mg/kg/24 h IV in 2 doses, not to exceed 2 g/24 h unless serum levels are monitored with or without gentamicin2 (1 mg/kg IV/IM every 8 h)
Pediatric Considerations
- Amoxicillin, 25-50 mg/kg/d in divided doses every 8 h
- Penicillin G , 100,000-250,000 U/kg/24 h IV/IM in divided doses every 4 h with or without gentamicin2 (1 mg/kg IM/IV every 6 h)
OR
- Ampicillin, 100-200 mg/kg/24 h IM/IV in 4-6 divided doses with or without gentamicin2 (1 mg/kg IV/IM every 6 h)
Penicillin Allergic/High-Level Penicillin Resistance, ß-Lactamase-Negative
- Nitrofurantoin, 50-100 mg orally every 6 h
OR
- Ofloxacin, 200 mg orally every 12 h
OR
- Ciprofloxacin, 250-500 mg orally every 12 h
OR
- Levofloxacin, 250 mg orally every d
OR
- Norfloxacin, 400 mg orally every 12 h
OR
- Enoxacin, 200-400 mg orally every 12 h
OR
- Gatifloxacin, 200-400 mg orally every d
- Vancomycin, 30 mg/kg/24 h IV in 2 doses, not to exceed 2 g/24 h unless serum levels are monitored with or without gentamicin2 (1 mg/kg IV/IM every 8 h)
Vancomycin and Penicillin Resistance
- Nitrofurantoin, 50-100 mg orally every 6 h
- Quinupristin/Dalfopristin, 7.5 mg/kg IV every 8 h
- Linezolid, 600 mg IV orally every 12 h
- Doses provided are for patients with normal renal function (creatinine clearance >70 mL/min). Abbreviations: IV, intravenously; IM, intramuscularly.
- For isolates without high-level gentamicin resistance.
BOX 3. Prevention of VRE Transmission
- All patients harboring VRE should be placed in private rooms or the same room as other patients who have VRE.
- Clean, nonsterile gloves should be worn when entering the room of a VRE-infected or -colonized patient.
- When caring for such a patient, a change of gloves is necessary after contact with material that could contain high concentrations of VRE (eg, stool).
- A gown should be worn when entering the room of a VRE patient if (a) substantial contact with the patient or with environmental surfaces in the patient’s room is anticipated; (b) the patient is incontinent; or (c) the patient has had an ileostomy or colostomy, has diarrhea, or has a wound drainage not contained by the dressing.
- Gloves and gowns should be removed before leaving the patient’s room, and the health care worker’s hands should be washed with antiseptic soap or waterless antiseptic agent.
- Dedicated noncritical items such as stethoscopes, sphygmomanometers, and rectal thermometers should be assigned to a single patient or cohort of patients infected or colonized with VRE.
- If such devices are to be used on other patients, they should be adequately cleaned and disinfected first.







