BORRELIA SPECIES
RELAPSING FEVER
Essentials of Diagnosis
- The most common presentation is fever with rash, headache, shaking chills, myalgias, arthralgias, and — during the acute phase — hepatosplenomegaly.
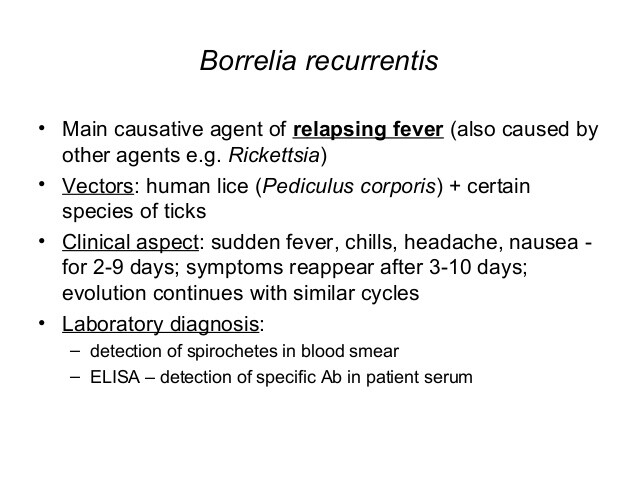
- Louse-borne relapsing fever (LBRF) is epidemic, caused by B. recurrentis, and characterized by one or two relapses.
- Tick-borne relapsing fever (TBRF) is endemic, caused by several Borrelia species, and characterized by multiple clinical relapses.
- Organisms can be visualized in blood smears of febrile patients, unlike other spirochetal pathogens, using dark-field microscopy or Giemsa or Wright stains.
- Helical (3-10 spirals) spirochetes, 8-30 um × 0.3 um, motile (flagella).
- Weil-Felix reaction: Proteus OX-K agglutinin titers are elevated (this is more common in LBRF).
General Considerations
The syndrome of relapsing fever consists of two clinical entities: epidemic relapsing fever caused by Borrelia recurrentis (LBRF) and transmitted by the human body louse and endemic relapsing fever caused by Borrelia spp. (TBRF) and transmitted by arthropods (Table 1). A. Epidemiology. 1. Louse-borne epidemic relapsing fever (LBRF). This form of relapsing fever is transmitted person to person.
Human body lice (Pediculus humanus corporis) ingest human blood infected with B recurrentis. The spirochetes multiply in the hemolymph of the lice. Humans become infected with B recurrentis when the fluids from a crushed louse contaminate mucous membranes or abrasions or other breaks in the skin. Humans are the only host for B recurrentis — its occurrence reflects socioeconomic and ecologic factors.
Endemic foci are still observed in parts of Africa, South America, and the Far East. 2. Tick-borne endemic relapsing fever (TBRF). TBRF is caused by = 15 different types of Borrelia spp. and is transmitted to humans by ticks (Ornithodoros spp.). The spirochete is capable of invading all tissues of the tick. Infections in humans occur when saliva or excrement is released while the tick is feeding. The ticks are nocturnal feeders and are rarely noticed. The tick’s bite does not cause pain, and feeding is completed after 5-20 min. Many rodents and small animals are reservoirs for Borrelia spp. The spirochete’s geographic distribution and occurrence is determined by the biology of the tick. Ticks carrying Ornithodoros spp. are mostly found at altitudes of 1500-6000 feet and in humid, warm climates. TBRF has been reported in Africa, Asia, South America, and the western United States during the summer. B. Microbiology. Borrelia spp. belong to the family Treponemataceae.
They are motile helical bacteria, 8-30 um long, 0.2-0.5 um wide, and with 3-10 loose spirals. Desiccation and UV light kill Borrelia spp. The generation time of these organisms is 18-26 h. They are not cultured in routine clinical laboratories but can be grown in chick embryos. C. Pathogenesis. After inoculation in humans from the tick or louse vector, an acute febrile illness ensues, accompanied by a spirochetemia.
The bacteria are present in the bloodstream only during the febrile illness. During the afebrile period the bacteria are sequestered in internal organs such as the central nervous system (CNS), bone marrow, liver, and spleen. There is a cycle of antigenic variation with specific antibody responses that explains the relapses of the disease. Each remission is the result of mobilization, opsonization by antibodies produced against the variant strain, agglutination, and phagocytosis of the organism by antibodies produced against the variant strain. Borreliae can cross the placenta and produce abortion or severe infection in neonates.
Clinical Findings
A. Signs and symptoms. LBRF and TBRF have similar signs and symptoms. The mean incubation period is 7 days (range, 2-18 days). Symptoms include sudden onset of fever, headache, photophobia, sweats, shaking chills, cough, nausea, vomiting, myalgias, and arthralgias (Box 1). Common findings include conjunctival suffusion, petechia, upper-quadrant abdominal tenderness, and hepatosplenomegaly. Other complications include gastrointestinal or CNS hemorrhage or both and myocarditis. A truncal rash at the end of the primary febrile episode with tachycardia is common.
The fever may be accompanied by hypotension and shock. Fever can reappear with less intensity after 10 days. LBRF has usually a single or few relapses; TBRF has multiple relapses. Each successive relapse of TBRF is milder and of shorter duration. Each intervening afebrile period is longer than the previous one. B. Laboratory findings. LBRF and TBRF are established by the detection of the spirochetes in blood from febrile patients.
Borrelia spp. can be detected in 70% of cases with dark-field microscopy, Giemsa-, Wright-, or acridine orange-stained preparations of blood smears or thick and thin smears. Serologies have limited value and are not standardized because of antigenic variation. Cross-reaction may occur with other spirochetes (5-10%).
Welix-Felix agglutination of Proteus OX-K in convalescent-phase serum supports the diagnosis. Leukocytosis, mild normocytic anemia, and thrombocytopenia are common. There may also be an elevated sedimentation rate, increased prothrombin and partial thromboplastin times, and an increase in aspartate aminotransferase and bilirubin.
Differential Diagnoses
LBRF and TBRF may be confused with malaria, typhoid fever, hepatitis, leptospirosis, salmonellosis, infectious mononucleosis, viral respiratory infections, rat bite fever, Colorado tick fever, Rocky Mountain spotted fever, and dengue fever. Epidemiologic features and detection of spirochetemia can help to exclude these diagnoses.
Treatment
Relapsing fever responds to doxycycline, tetracycline, erythromycin, penicillin, and chloramphenicol (Box 2). One single dose of 500 mg of tetracycline (or erythromycin in children, pregnant patients, or patients with penicillin allergies) for LBRF and a 10-day course for TBRF are usually satisfactory. Antibiotic treatment can induce a Jarisch-Herxheimer reaction within 2 h; this reaction coincides with clearing of the spirochetes. This reaction is not prevented with corticosteroids.
Prognosis
With appropriate therapy, mortality rates from relapsing fever are < 5%. In untreated patients the fatality rate for LBRF may reach 40%, and in TBRF is = 5%.
Prevention
LBRF can be prevented with good personal hygiene, delousing procedures, and secondary prevention with case detection and treatment (Box 3). TBRF requires avoidance or elimination of the tick, using acaricides.
Lyme Disease
LEPTOSPIRA SPECIES
Essentials of Diagnosis
- The most severe forms of leptospirosis commonly present with liver and renal involvement.
- Transmission occurs by indirect contact with an infected animal.
- Predisposing factors include occupational exposure (veterinarians and farmers) and recreational exposure (campers and swimmers).
- These organisms can be detected by dark-field examination, silver or fluorescent antibody stains, or PCR. Tween 80-albumin is the best medium for culture.
- Motile spirochete, 0.1 x 6-20 um, with hooked end.
- Isolation from any clinical specimen or seroconversion or fourfold increase in antibody titers is diagnostic.
General Considerations
Leptospirosis is caused by multiple species of Leptospira and is characterized by two different forms of disease. There is a mild form (anicteric) and a severe form (icteric, also known as Weil’s syndrome) of leptospirosis.
Epidemiology
Leptospirosis is a zoonosis of worldwide distribution and is especially common in tropical regions. Rodents, dogs, cats, other wild mammals, fish, and birds are important reservoirs. The bacteria can live in the renal tubules of dogs for long periods of time.
Transmission to humans occurs by indirect contact with urine, blood, or tissue of an infected animal (eg, by veterinarians or farmers); human-to-human transmission is rare. It can be contracted during recreational activities involving water. Leptospirosis has been reported during the summer and autumn months in the southern and western United States and especially in Hawaii. B. Microbiology. Leptospires are spirochetes belonging to the genus Leptospira, which is composed of two species: L interrogans and L biflexa.
The pathogenic leptospires belong to the first species and are divided into 200 serotypes that have major antigens in common and are combined into 23 serogroups. Leptospires are motile spirochetes, 0.1 um wide, 6-20 um long, and with hooked ends. C. Pathogenesis.
The bacteria enter the abraded skin or mucous membranes and spread by blood to multiple organs including liver (liver disease occurs in severe cases due to hepatocellular dysfunction including decreased production of clotting factors and albumin, and reduced esterification of cholesterol), kidneys (renal failure due to tubular damage by immune complexes, hypoxemia, or direct toxic effect of the leptospires), CNS (in the first week, the leptospira can be found in the CSF, but no meningitis is seen until the serum antibody appears), eye (can produce chronic or recurrent uveitis), muscle (changes include cytoplasmic vacuoles and polymorphonuclear leukocyte infiltration), and blood vessels (vasculitis, capillary injury, and hemolysis are characteristic features).
Clinical Findings
Signs and symptoms
Leptospirosis has an incubation period of 1-2 weeks. Among infected patients who develop leptospirosis, 90% have the anicteric form, and 10% have the icteric form. There are two phases — septic and immune. The septic phase lasts 4-7 days and consists of a flulike syndrome; during this phase leptospiras can be found in the bloodstream. The immune phase lasts 4-30 days and consists of aseptic meningitis, uveitis, iritis, rash, and hepatic and renal involvement. During this phase, leptospiras can be found in urine and aqueous humor (Box 7). 1. Anicteric leptospirosis. The septic phase is characterized by fever, headache, myalgias, abdominal pain, nausea, and vomiting. The immune phase consists of less prominent fever, intense headache, aseptic meningitis, conjunctival suffusion, uveitis, hepatosplenomegaly, pulmonary involvement, and skin rashes. 2. Icteric leptospirosis. The septic phase resembles that of the anicteric leptospirosis. The most prominent manifestation during the immune phase is hepatorenal dysfunction with hemorrhagic diathesis.
Laboratory findings
Laboratory findings in anicteric leptospirosis include normal leukocyte counts with neutrophilia and elevated ESR and CSF protein. Pulmonary and myocardial involvement; high bilirubin levels; increases in alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, creatine phosphokinase, creatinine, and blood urea nitrogen; and thrombocytopenia are commonly found in icteric leptospirosis. The diagnosis is made by isolation of the organism from any clinical specimen or seroconversion or fourfold increase in antibody titer.
The bacteria can be isolated from blood or CSF during the first 10 days. Tween 80-albumin medium is preferred, and multiple cultures should be performed. The organisms can also be detected by dark-field examination, PCR, silver stains of body fluids or fluorescent antibody stains of tissue. Leptospira-specific antibodies can be detected by macroscopic agglutination with killed antigen, microscopic agglutination with live antigen (more specific) and enzyme-linked immunosorbent assay. Agglutinins appear after 6-12 days, and peak titers are reached in 3-4 weeks. Cross-reaction is common with other spirochetal diseases.
Differential Diagnoses
Differential diagnoses include dengue, dengue fever, hemorrhagic fever, LBRF, TBRF, and other diseases caused by arthropod-borne and rodent-borne pathogens.
Complications
Aseptic meningitis is the most common complication in the anicteric cases. Renal failure, liver damage, pulmonary hemorrhage, vasculitis, and myocarditis are less common but are the usual causes of death.
Treatment
Penicillin G or doxycycline are effective even when treatment is delayed (Box 8). Penicillin G or ampicillin should be used in severely ill patients. In less severely ill patients, an oral dose of ampicillin, doxycycline, or amoxicillin can be used.
Prognosis
In the absence of jaundice, the disease is rarely fatal. The mortality rate in icteric patients under 30 years of age is 5%; in the elderly the rate is 30-40%.
Prevention
Doxycycline can prevent infection (Box 9). However, prevention is problematic because exposure is difficult to predict. Effective rat control and avoidance of infected urine and known contaminated water sources are important preventive measures.
Stage Clinical manifestation Other diagnoses Localized Infection
- Erythema chronicum migrans
- Streptococcal cellulitis
- Erythema multiforme
- Erythema marginatum
- Tinea corporis (ringworm)
- Nummular eczema
- Granuloma annulare
Disseminated Infection
- 7th nerve palsy
- Idiopathic Bell palsy
- CNS tumor
- Myocarditis (viral and other etiologies)
- Acute rheumatic fever
- Carditis
- Endocarditis
- Meningitis
- Viral meningitis
- Parameningeal infections
- Postinfectious meningoencephalitis
- Leptospiral meningitis
- Tuberculous meningitis
- Listeria partially treated
- Bacterial (pyogenic) meningitis
- Subacute (to chronic) meningitis
- Arthritis
- Acute rheumatic fever
- Malignant effusion
- Post-traumatic effusion
- Hemophilia
- Pyogenic arthritis
Persistent Infection
- Fever
- Rash
- Headache
- Shaking chills
- Nausea, vomiting
- Myalgias
- Arthralgias
- Cough
- Conjunctival injection
- Petechia (more in LBRF)
- Hepatosplenomegaly
- Single relapse with LBRF
- Multiples relapses with TBRF
Less Common
- Nuchal rigidity (meningitis)
- Pulmonary rales, ronchi, pleuritic pain
- Lymphadenopathy
- Jaundice
- Gastrointestinal and CNS hemorrhage
- Myocarditis
- Rupture of the spleen
LBRF (single dose) TBRF (7 day course) First Choice
- Doxycycline 100 mg
- 100 mg at 12-h intervals
Second Choice
- Tetracycline 500 mg
- Erythromycin 500 mg
- Chloramphenicol 500 mg
- Penicillin G (procaine) 600,000 IU
- 500 mg at 6-h intervals
- 500 mg at 6-h intervals
- 500 mg at 6-h intervals
- 600,000 IU daily
Pediatric Considerations
- Erythromycin 40 mg/kg/day
- Penicilin G (procaine) 10,000 IU/kg/day
- 40 mg/kg/day
- 10,000 IU/kg/day
Penicillin Allergic
- Tetracycline 500 mg
- Erythromycin 500 mg
- 500 mg at 6-h intervals
- 500 mg at 6-h intervals
Antibiotic treatment can induce a Jarish-Herxheimer reaction within 2 h and coincides with clearing of the spirochetes. 2LBRF, louse-borne relapsing fever: TBRF, tick-borne relapsing fever Tetracycline should be avoided in children < 9 years of age.
LBRF TBRF Prophylactic Measures
- Good personal hygiene
- Delousing procedures
- Improving socioeconomic conditions (crowding, poverty, homelessness)
- Wear cloth to protect skin
- Avoid rodent- and tick-infested dwellings
- Pest control, repellents, acaricide
LBRF, louse-borne relapsing fever; TBRF, tick-borne relapsing fever.
BOX 4. Systems Affected in Lyme Disease (in Children and Adults)
More Common
- Skin: Erythema chronicum migrans and acrodermititis chronica atrophicans
- Joints: Asymmetric monoarticular or oligoarticular arthritis
- CNS: Facial palsy, peripheral neuritis, encephalitis, cerebral vasculitis, and chronic encephalopathy
- Cardiac: AV blocks of various degrees
Less Common
- Eye: Conjuctivitis, keratitis, uveitis, retinitis, and optic neuritis
- Liver: Hepatitis
- Lung: Adult respiratory distress syndrome
- Congenital: Intrauterine fetal death, prematurity, cortical blindness, or no adverse outcome
CNS, central nervous systems; AV, atreoventricular.
BOX 5. Treatment of Lyme Disease in Children and Adults
Oral Therapy Intravenous Therapy First Choice
- Doxycycline, 100 mg twice per day
- Amoxicillin, 500 mg three times per day
- Ceftriaxone, 2000 mg daily
Second Choice
- Clarithromycin, 500 mg twice per day
- Azithromycin, 500 mg daily
- Cefuroxime, 500 mg twice per day
- Cefotaxime, 2000 mg at 8-h intervals
- Penicillin G, 5 million IU at 6-h intervals
Pediatric Considerations
- Amoxicillin, 50 mg/kg/day
- Ceftriaxone, 75 mg/kg/day
- Penicillin G, 300,000 IU/kg/day
Penicillin Allergic
- Clarithromycin, 500 mg twice per day
- Azithromycin, 500 mg daily
- Doxycycline, 100 mg twice per day
- Azithromycin, 500 mg daily
Tetracycline should be avoided in children < 9 years of age.
BOX 6. Control of Lyme disease
Prophylactic Measures
- Doxycycline, 100 mg twice per day by mouth
- Amoxicillin, 500 mg at 8-h intervals per 10 days by mouth
- Vaccine in high-risk workers and persons living or visiting endemic areas
Isolation Precautions
- Wear cloth to protect skin
- Repellents, insecticides
- Check for ticks every 24 h
BOX 7. Leptospirosis in Children and Adults
More Common Anicteric
- Septic phase: (3-7 d) Fever, headache, myalgias, abdominal pain, nausea, vomiting
- Immune phase: (0 d-1 mo) Lower fever, intense headache, aseptic meningitis, conjuctival injection, uveitis, hepatosplenomegaly, pulmonary involvement, skin rashes
Less Common Icteric
- Septic phase: (3-7 d)
- Imune phase: (10-30 d) Jaundice, renal dysfunction, vasculitis, pulmonary hemorrhage, myocarditis
BOX 8. Treatment of Leptospirosis in Children and Adults
Anicteric Icteric First Choice
- Ampicillin, 500 mg four times daily by mouth
- Amoxicillin, 500 mg four times daily by mouth
- Doxycycline, 100 mg four times daily by mouth
- Penicillin G, 1.5 million IU four times daily intravenously
- Ampicillin, 1000 mg four times daily intravenously
- Amoxicillin, 1000 mg four times daily intravenously
Second Choice
- Tetracycline, 2000 mg/day by mouth
- Erythromycin,2 500 mg four times daily intravenously
Pediatric Considerations
- Ampicillin, 50 mg/kg/day by mouth
- Amoxicillin, 50 mg/kg/day by mouth
- Penicillin G, 6-8 million IU/m2 day intravenously
- Erythromycin,2 50 mg/kg/day intravenously
Penicillin Allergic
- Doxycycline, 100 mg four times daily by mouth
- Erythromycin,2 500 mg four times daily intravenously
Tetracycline should be avoided in children < 9 years of age. 2Erythromycin is active in in-vitro and animal models but no human clinical data is available.
BOX 9. Control of leptospirosis
Prophylactic Measures
- Doxycycline 200 mg once a week
Isolation Precautions
- Effective rat control
- Avoidance of infected urine and tissues from animals
- Vaccination of animals