Drug Nomenclature
International Nonproprietary Names (INNs) in main languages (French, Latin, Russian, and Spanish):
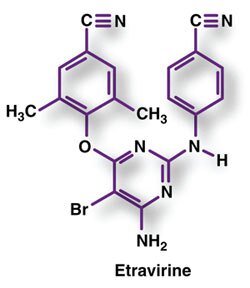
Etravirina; Étravirine; Etravirinum; R-165335; TMC-125.
4-[6-Amino-5-bromo-2-(4-cyanoanilino)pyrimidin-4-yloxy]-3,5-dimethylbenzonitrile.
Этравирин
C20H15BrN6O = 435.3.
CAS — 269055-15-4.
Adverse Effects
The most common adverse effects associated with antiretroviral regimens containing etravirine are nausea and skin rash (usually mild to moderate) and generally appearing in the second week of treatment and resolving within 1 to 2 weeks. Severe skin reactions, including erythema multiforme and Stevens-Johnson syndrome, have occurred.
Additional adverse events of moderate to severe intensity reported by at least 2% of patients receiving etravirine in clinical studies included gastrointestinal complaints (abdominal pain, diarrhoea, nausea, and vomiting), fatigue, headache, hypertension, and peripheral neuropathy.
Raised liver enzyme values, glucose levels, and serum-cholesterol and -triglyceride concentrations have been reported. Immune reconstitution syndrome (an inflammatory immune response resulting in clinical deterioration) has been reported during the initial phase of treatment with combination antiretroviral therapy, including etravirine, in HIV-infected patients with severe immune deficiency.
Accumulation or redistribution of body fat (lipodystrophy) including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and cushingoid appearance have been seen in patients receiving antiretroviral therapy, including etravirine.
Precautions
Etravirine should be stopped if a severe skin rash develops. Patients co-infected with chronic hepatitis B or C have experienced worsening of hepatitis-related symptoms when treated with etravirine. Patients who have virologic failure on a NNRTI-containing regimen should not be given etravirine in a regimen containing only NRTIs.
Interactions
Etravirine is metabolised mainly by the cytochrome P450 isoenzymes CYP3A4, CYP2C9, and CYP2C19. It is an inducer of CYP3A4 and an inhibitor of CYP2C9 and CYP2C19. Consequently it may compete with other drugs metabolised by these systems, potentially resulting in mutually altered plasma concentrations and possibly toxicity. Enzyme inducers may decrease plasma concentrations of etravirine.
Etravirine should not be given with other NNRTIs. It should also not be used in regimens with HIV-protease inhibitors given without ritonavir-boosting but use with ritonavir-boosted tipranavir, fosamprenavir, or atazanavir should be avoided. For further information on drug interactions of NNRTIs see site.
Antiviral Action
Etravirine acts by inhibition of HIV-1 reverse transcriptase and blocks viral RNA- and DNA-dependent DNA polymerase activities. It is a flexible molecule designed to fit in the active pocket of viral reverse transcriptase in different ways, even when the shape of that pocket changes because of viral mutations. This is considered to reduce the risk of the development of resistance; phase II studies in treatment-experienced patients have shown activity against HIV resistant to other NNRTIs (delavirdine, efavirenz, and nevirapine).
Pharmacokinetics
Etravirine is readily absorbed after oral doses and peak plasma concentrations occur after about 2.5 to 4 hours; absorption is increased by food. It is about 99.9% bound to plasma proteins. Etravirine is extensively metabolised by hepatic microsomal enzymes, principally by the cytochrome P450 isoenzymes CYP3A4, CYP2C9, and CYP2C19 families, to substantially less active metabolites. The mean plasma half-life after usual dosage is about 41 hours and ranges from 21 to 61 hours. About 93.7% of a dose appears in the faeces (81.2 to 86.4% as unchanged drug), and 1.2% in the urine (unchanged drug was not detected in the urine).
Uses and Administration
Etravirine is a non-nucleoside reverse transcriptase inhibitor with activity against HIV-1. It is given with other antiretrovirals for the treatment of HIV infection and AIDS in treatment-experienced patients, who have evidence of viral replication and HIV-1 strains resistant to a NNRTI and other antiretrovirals. Etravirine is given orally in a usual dose of 200 mg twice daily after food.
Proprietary Preparations
USA: Intelence

