Drug Approvals
Synonyms: CI-898 (trimetrexate); JB-11 (trimetrexate); NSC-249008 (trimetrexate); NSC-328564 (trimetrexate); NSC-352122; Trimetrexato, glucuronato de
BAN: Trimetrexate Glucuronate [BANM]
USAN: Trimetrexate Glucuronate
INN: Trimetrexate Glucuronate [rINNM (en)]
INN: Glucuronato de trimetrexato [rINNM (es)]
INN: Trimétrexate, Glucuronate de [rINNM (fr)]
INN: Trimetrexati Glucuronatum [rINNM (la)]
INN: Триметрексата Глюкуронат [rINNM (ru)]
Chemical name: 5-Methyl-6-(3,4,5-trimethoxyanilinomethyl)quinazolin-2,4-diyldiamine mono-d-glucuronate
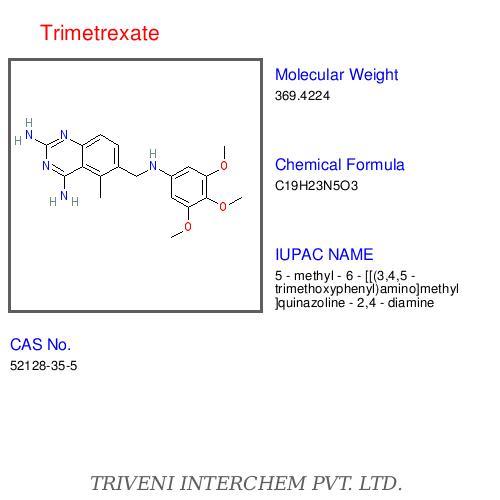
Molecular formula: C19H23N5O3,C6H10O7 =563.6
CAS: 52128-35-5 (trimetrexate); 82952-64-5 (trimetrexate glucuronate)
ATC code: P01AX07
Read code: y09uR
Incompatibility
Trimetrexate is reported to be incompatible with foscarnet. Trimetrexate should not be mixed with folinic acid or chloride ions, since precipitation occurs instantly.
Adverse Effects, Treatment, and Precautions
Trimetrexate is a dihydrofolate reductase inhibitor and therefore adverse effects and precautions are similar to those of methotrexate. It must be given with folinic acid, which should be continued for 72 hours after the last dose of trimetrexate.
Interactions
Studies in animals suggest that cimetidine and imidazole antifungals such as clotrimazole and ketoconazole may inhibit trimetrexate metabolism, and there is a risk of possible interactions with all drugs that affect hepatic cytochrome P450 systems.
Antimicrobial Action
Trimetrexate is an inhibitor of dihydrofolate reductase and consequently prevents formation of the active coenzyme tetrahydrofolate and production of DNA and RNA precursors, leading to cell death. At therapeutic doses the selective transport of trimetrexate, but not folinic acid, into Pneumocystis jirovecii allows folinic acid to protect normal host cells from the cytotoxicity of trimetrexate without inhibiting its antifungal activity. In-vitro trimetrexate has shown dose-related inhibition of growth of the trophozoite stage of P. jirovecii.
Pharmacokinetics
The pharmacokinetics of intravenous trimetrexate have been described as both biphasic and triphasic, with a terminal elimination half-life of about 16 to 18 hours. After use with folinic acid a biphasic disposition with a terminal half-life of 11 hours has also been reported. It is extensively protein bound reports suggest that it is 95 to 98% bound at low serum concentrations, but that binding is saturable, with free fraction increasing at plasma concentrations above 1 microgram/mL. Trimetrexate is excreted mainly in the urine, as unchanged drug and metabolites, some of which may be active. The major metabolic pathway appears to be oxidative O-demethylation followed by conjugation to the sulfate or glucuronide.
Uses and Administration
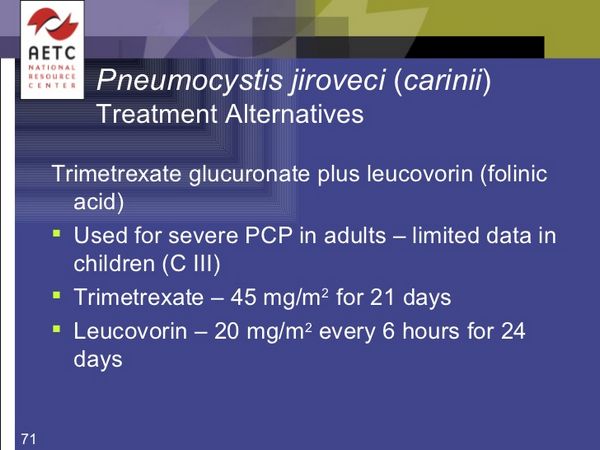
Trimetrexate is a dihydrofolate reductase inhibitor with general properties similar to those of methotrexate. It is used in the management of moderate to severe pneumocystis pneumonia in immunocompromised patients, notably patients with AIDS, where other therapy has proved ineffective. It has also been tried as an antineoplastic in the management of various solid tumours. Trimetrexate is given as the glucuronate but doses are stated in terms of trimetrexate. Trimetrexate glucuronate 1.53 mg is equivalent to about 1 mg of trimetrexate. It is given by intravenous infusion, over 60 to 90 minutes. The schedule in pneumocystis pneumonia is 45 mg/m daily for 21 days, in association with folinic acid rescue for 24 days. The dosage of trimetrexate and folinic acid should be adjusted according to the results of blood tests, which should be performed at least twice a week during therapy. Renal and hepatic function and haemoglobin values should also be monitored. Treatment with zidovudine and other myelosuppressive drugs should be interrupted to allow full doses of trimetrexate to be given.
Proprietary Preparations
Hong Kong: Neutrexin
Ireland: Neutrexin
Spain: Neutrexin
Thailand: Neutrexin
USA: Neutrexin

