Drug Nomenclature
Pharmacopoeias in China, Europe, International, Japan, and US
European Pharmacopoeia, 6th ed. (Flucytosine)
A white or almost white crystalline powder. Sparingly soluble in water slightly soluble in dehydrated alcohol. Protect from light.
The United States Pharmacopeia 31, 2008 (Flucytosine)
A white to off-white crystalline powder, odourless or with a slight odour. Sparingly soluble in water slightly soluble in alcohol practically insoluble in chloroform and in ether. Store in airtight containers. Protect from light.

Stability
A solution of flucytosine for intravenous infusion should be stored between 18° and 25°. Precipitation may occur at lower temperatures and decomposition, with the formation of fluorouracil, at higher temperatures.
Adverse Effects
Adverse effects of flucytosine include nausea, vomiting, diarrhoea, and skin rashes. Less frequently reported adverse effects include confusion, hallucinations, convulsions, headache, sedation, and vertigo, and also allergic reactions, toxic epidermal necrolysis, and car-diotoxicity. Alterations in liver function tests are generally dose-related and reversible hepatotoxicity may also occur.
Hypokalaemia may occur.
There have been a few reports of peripheral neuropathy. Bone-marrow depression, especially leucopenia and thrombocytopenia, is associated with blood concentrations of flucytosine greater than 100 micrograms/mL, with concurrent use of amphotericin B, and with renal impairment. Fatal agranulocytosis and aplastic anaemia have been reported.
Effects on the blood
Bone marrow toxicity associated with flucytosine has been attributed to its conversion to fluorouracil, possibly by intestinal flora. A pilot study of 6 patients given intravenous flucytosine found that the amounts of fluorouracil in serum samples were undetectable, whereas flucytosine could be detected in the samples. This might be because intravenous dosage did not allow the conversion of flucytosine to fluorouracil by intestinal microflora. However, one patient still developed thrombocytopenia and another leucocytopenia and the authors hypothesised that toxicity might be due to flucytosine and not the metabolite.
Precautions
Flucytosine should be given with great care to patients with renal impairment, or with blood disorders or bone marrow depression. Renal and hepatic function and blood counts should be monitored during therapy (at least weekly in patients with renal impairment or blood disorders). In patients with renal impairment, doses should be reduced and trough blood concentrations of flucytosine should be checked regularly from blood samples taken just before an injection of flucytosine (see under Uses, below). Care should be taken in patients given radiation therapy or other drugs which depress bone marrow. Flucytosine is teratogenic in rats.
AIDS
Frequent bone marrow toxicity has been reported in patients with AIDS during flucytosine therapy. However, in a study in 3 81 patients, no additional haematotoxicity was reported in patients given amphotericin B plus flucytosine compared with those given amphotericin B alone. The toxicity could be minimised by monitoring serum concentrations and the British Society for Antimicrobial Chemotherapy has suggested that these should be maintained within 25 to 50 micrograms/mL in patients with AIDS.
Pregnancy
Teratogenicity has been reported in some animal models and licensed product information recommends that flucytosine should only be used if the benefit justifies that possible risk to the fetus. The congenital defects are thought to be as a result of the conversion of flucytosine to fluorouracil by the intestinal microflora. However, there are some case reports of pregnant patients receiving flucytosine (with or without amphotericin B) in the second and third trimesters with no reports of abnormalities in the infants.
Interactions
Flucytosine is commonly used with amphotericin B. Amphotericin B can cause a deterioration in renal function, which can result in raised flucytosine blood concentrations and increased toxicity. However, the two drugs are generally regarded as having synergistic antifungal activity. Cytarabine has been claimed to reduce blood concentrations of flucytosine and to antagonise its antifungal activity, although the evidence is limited.
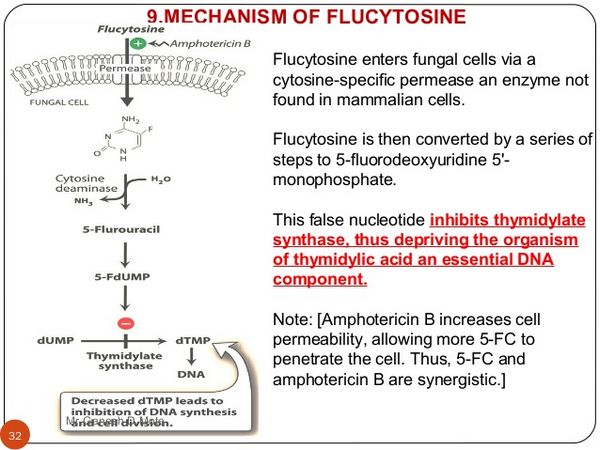
Antimicrobial Action
Flucytosine is a fluorinated pyrimidine antifungal. In susceptible fungi it is converted by cytosine deaminase to fluorouracil which is then incorporated in place of uracil into fungal RNA and disrupts protein synthesis. The activity of thymidilate synthetase is also inhibited and this effect interferes with fungal DNA synthesis. Flucytosine is active against Candida spp., Cryptococcus neoformans, Cladosporium spp., and Fonsecaea spp. Some Aspergillus spp. have also been reported to be sensitive. There is synergy between flucytosine and amphotericin B against Candida spp. and Cryptococcus neoformans. There is a high incidence of primary resistance to flucytosine among isolates of Candida spp. and Cryptococcus neoformans. Resistance also develops during treatment with flucytosine and has been reported rarely from combination therapy with flucytosine and amphotericin B.
Pharmacokinetics
Flucytosine is absorbed rapidly and almost completely from the gastrointestinal tract. Bioav ail ability is 78 to 89%. After oral doses of 37.5 mg/kg every 6 hours, peak plasma concentrations of 70 to 80 micrograms/mL have been achieved within 2 hours similar concentrations have been achieved but more rapidly, after an intravenous dose. The plasma-flucytosine concentration for optimum response is 25 to 50 micrograms/mL.
Flucytosine is widely distributed through the body tissues and fluids concentrations in the CSF are 65 to 90% of those in serum. About 2 to 4% of flucytosine is protein bound. About 90% of a dose is excreted unchanged by glomerular filtration a small amount of flucytosine may be metabolised to fluorouracil. The small amount of an oral dose of flucytosine not absorbed from the gastrointestinal tract is eliminated unchanged in the faeces. The elimination half-life is 2.5 to 6 hours in patients with normal renal function but increases with decreasing renal function. Flucytosine is removed by haemodialysis or peritoneal dialysis.
Uses and Administration
Flucytosine is a fluorinated pyrimidine antifungal used in the treatment of systemic fungal infections, the treatments for which are discussed under Choice of Antifungal. It is mainly used with amphotericin B or fluconazole in the treatment of severe systemic candi-diasis and cryptococcal meningitis. It has also been tried in other infections due to susceptible fungi including chromoblastomycosis. Flucytosine is given by intravenous infusion as a 1% solution over 20 to 40 minutes. The usual dose is 200 mg/kg daily in 4 divided doses a dose of 100 to 150 mg/kg daily may be sufficient in some patients. Dosage should be adjusted to produce trough plasma concentrations of 25 to 50 micrograms/mL. This is particularly important in patients with AIDS who are at increased risk of bone marrow toxicity.
Parenteral treatment is rarely given for more than 7 days, except for cryptococcal meningitis when it is continued for at least 4 months. For intravenous doses to be used in patients with renal impairment, see below. Flucytosine is given orally in usual doses of 50 to 150 mg/kg daily in 4 divided doses. Again, blood concentrations should be monitored and dosage adjusted in patients with renal impairment to avoid accumulation of the drug (see below). Flucytosine has been used topically for azole-refractory vaginitis caused by Candida spp., but such use may increase problems of resistance.
Administration in renal impairment
Flucytosine is mainly excreted by the kidneys and the dose must be adjusted in patients with renal impairment. Dose intervals for intravenous flucytosine should be adjusted according to creatinine clearance (CC): For intravenous use
- CC 20 to 40 mL/minute: 50 mg/kg every 12 hours
- CC 10 to 20 mL/minute: 50 mg/kg every 24 hours
- CC less than 10 mL/minute: 50 mg/kg then further doses should be based on plasma concentrations which should not exceed 80 micrograms/mL
Initial oral doses should be at the lower end of the recommended range (see above) and dosage should be adjusted subsequently to avoid accumulation.
Preparations
British Pharmacopoeia 2008: Flucytosine Tablets
The United States Pharmacopeia 31, 2008: Flucytosine Capsules Flucytosine Oral Suspension.
Proprietary Preparations
Argentina: Ancotil Australia:: Ancotil Austria: Ancotil Denmark: Ancotil France: An-cotil Germany: Ancotil Greece: Ancotil Hong Kong: Ancotil Italy: Ancotil Malaysia: Ancotil The Netherlands: Ancotil New Zealand: Alcobon Ancotil Poland: Ancotil Russia: Ancotyl Singapore: Ancotil Sweden: Ancotil Switzerland: Ancotil UK: Ancotil USA: Ancobon

