Structure of the four main anthracyclines approved for clinical use
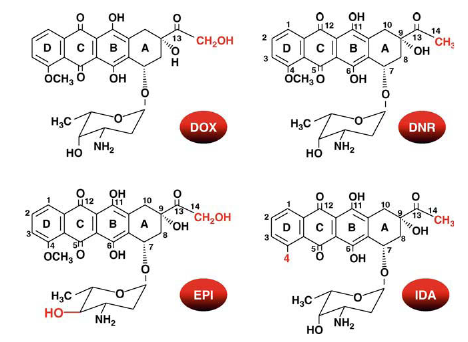
The anthracyclines are composed of a tetracyclic ring with adjacent quinone-hydroquinone moieties and a short side chain with a carbonyl group at C-13; an aminosugar is attached by a glycosidic bond to the C-7 of the tetracyclic ring. Doxorubicin (DOX) and daunorubicin (DNR) differ in the side chain terminus (-CH2OH or-CH3, respectively). Epirubicin (EPI) is obtained after an axial-to-equatorial epimerization of the hydroxyl group at C-4′ in the aminosugar. Idarubicin (IDA) is characterized by the absence of the methoxy group at C-4 in ring D.
Mechanisms of anthracycline-induced apoptosis of tumor cells
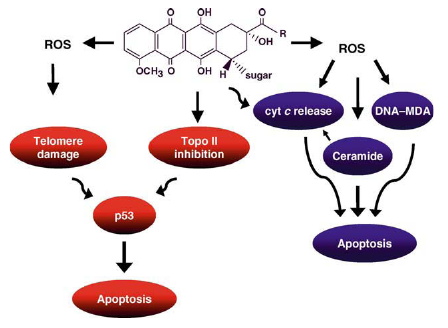
Mechanisms of anthracycline cardiotoxicity
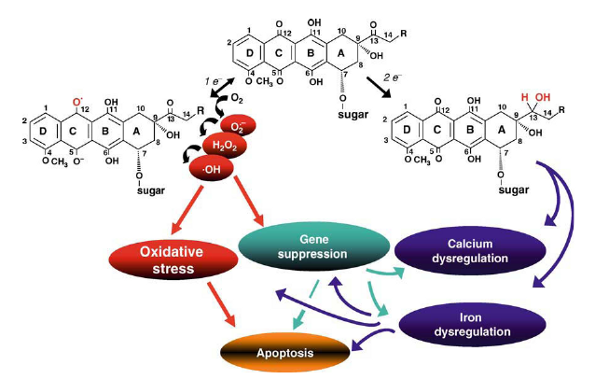
One-electron (7 e~) reduction of the quinone moiety generates a semiquinone that recycles to the parent quinone by redox coupling with oxygen. The consequent cascade of superoxide anion (O2~), hydrogen peroxide (H2O2), and hydroxyl radical (‘OH), induces apoptosis through oxidative stress and gene suppression. Two-electron (2 e~) reduction of the side chain carbonyl group generates a secondary alcohol metabolite that also suppresses cardiac-specific genes and inactivates proteins of calcium and iron homeostasis. These mechanisms share multiple links and feedbacks.


