Aminopenicillins GS, Extended-Spectrum Penicillins GS, Natural Penicillins GS, Penicillinase-Resistant Penicillins GS
Classification of Penicillins
Based on Spectra of Activity Penicillins are natural or semisynthetic antibiotics produced by or derived from certain species of the fungus Penicillium. The drugs are b-lactam antibiotics structurally and pharmacologically related to other b-lactam antibiotics including cephalosporins and cephamycins. Penicillins contain a 6-aminopenicillanic acid (6-APA) nucleus, which is composed of a b-lactam ring fused to a 5-membered thiazolidine ring. Although the 6-APA nucleus has little antibacterial activity itself, it is a major structural requirement for antibacterial activity of penicillins.
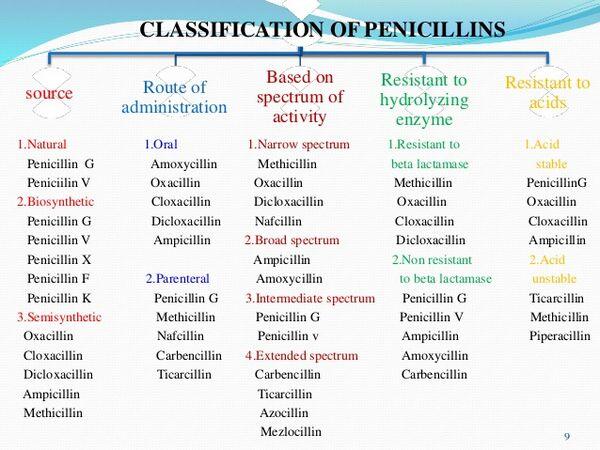
In currently available penicillins, cleavage at any point in the penicillin nucleus, including the b-lactam ring, results in complete loss of antibacterial activity. A free carboxyl group in the thiazolidine ring and one or more substituted amino side chains at R are also essential for antibacterial activity. penicillin nucleus A = b-lactam ring B = thiazolidine ring Addition of various side chains at R on the penicillin nucleus results in penicillin derivatives with differences in spectra of activity, stability against hydrolysis by b-lactamases, acid stability, GI absorption, and protein binding. Currently available penicillins can be divided into 4 groups based principally on their spectra of activity:62, 63, 64, 66, 575, 2537
Natural Penicillins
Penicillinase-Resistant Penicillins
Aminopenicillins
Extended-Spectrum Penicillins
Natural penicillins are produced by fermentation of mutant strains of Penicillium chrysogenum. Natural penicillins with different side chains at R are produced by altering the composition of the culture media of Penicillium. Although various natural penicillins have been produced (e.g., penicillins F, G, N, O, V, X), only penicillin G and penicillin V currently are used clinically.
Natural penicillins are active in vitro against many gram-positive aerobic cocci including most strains of nonpenicillinase-producing Staphylococcus aureus and S. epidermidis, Streptococcus pneumoniae, groups A, B, C, G, H, K, L, and M streptococci, nonenterococcal group D streptococci, viridans streptococci, and some strains of enterococci.
Natural penicillins are readily hydrolyzed by staphylococcal penicillinases and are therefore inactive against penicillinase-producing strains of S. aureus and S. epidermidis. The drugs are active in vitro against some gram-positive aerobic bacilli including Corynebacterium diphtheriae, Listeria monocytogenes, and Bacillus anthracis. Natural penicillins also are active in vitro against gram-negative aerobic cocci including Neisseria meningitidis and most strains of nonpenicillinase-producing N. gonorrhoeae.
The drugs are active in vitro against some gram-negative aerobic bacilli including some strains of Haemophilus influenzae, Pasteurella multocida, Streptobacillus moniliformis, and Spirillum minus. However, Pseudomonas and most Enterobacteriaceae are resistant to natural penicillins. Natural penicillins are active in vitro against many gram-positive anaerobic bacteria and some gram-negative anaerobic bacteria. The drugs generally are active in vitro against Actinomyces israelii, Peptococcus, Peptostreptococcus, Fusobacterium, Veillonella, and some strains of Bacteroides and Clostridium. The drugs also are active against most spirochetes, including Treponema pallidum, T. pertenue, Leptospira, Borrelia recurrentis, and B. burgdorferi, the causative agent of Lyme disease.
PENICILLINASE-RESISTANT PENICILLINS dicloxacillin nafcillin oxacillin
Penicillinase-resistant penicillins are semisynthetic derivatives of 6-APA that are stable against hydrolysis by most staphylococcal penicillinases. These penicillins have bulky side chains at R on the 6-APA nucleus that result in steric hindrance and help to prevent attachment of penicillinases to the b-lactam ring.
Because penicillinase-resistant penicillins are not hydrolyzed by most staphylococcal penicillinases, these drugs are active in vitro against many penicillinase-producing strains of S. aureus and S. epidermidis that are resistant to natural penicillins, aminopenicillins, and extended-spectrum penicillins.
Penicillinase-resistant penicillins also have some in vitro activity against other gram-positive bacteria and some gram-negative bacteria and spirochetes; however, the drugs generally are less active on a weight basis against these organisms than natural penicillins, and use of penicillinase-resistant penicillins generally is limited to the treatment of infections caused by susceptible penicillinase-producing staphylococci.
AMINOPENICILLINS amoxicillin ampicillin
Aminopenicillins are semisynthetic derivatives of 6-APA which have a free amino group at the a-position at R on the penicillin nucleus. Partly because of this polar group, aminopenicillins have enhanced activity against gram-negative bacteria compared with natural penicillins and penicillinase-resistant penicillins.
In vitro, aminopenicillins are generally active against gram-positive aerobic cocci and gram-positive aerobic bacilli that are susceptible to natural penicillins. However, with the possible exception of enterococcal infections, natural penicillins are generally the penicillins of choice for the treatment of infections caused by gram-positive cocci that are susceptible to both natural penicillins and aminopenicillins.
Like natural penicillins and extended-spectrum penicillins, aminopenicillins are readily hydrolyzed by staphylococcal penicillinases and are therefore inactive against penicillinase-producing strains of S. aureus and S. epidermidis. Aminopenicillins are generally active in vitro against gram-negative aerobic cocci, gram-negative aerobic bacilli, and anaerobic bacteria that are susceptible to natural penicillins. In addition, aminopenicillins are active in vitro against some Enterobacteriaceae including some strains of Escherichia coli, Proteus mirabilis, Salmonella, and Shigella.
Aminopenicillins are generally inactive against other Enterobacteriaceae, Bacteroides fragilis, and Pseudomonas. Because clavulanic acid and sulbactam can inhibit certain b-lactamases that generally inactivate aminopenicillins, combinations of amoxicillin and clavulanate potassium and combinations of ampicillin sodium and sulbactam sodium are active in vitro against many b-lactamase-producing organisms that are resistant to the aminopenicillins alone.
EXTENDED-SPECTRUM PENICILLINS carbenicillin piperacillin ticarcillin
Extended-spectrum penicillins are semisynthetic derivatives of 6-APA which have a wider spectra of activity than natural penicillins, penicillinase-resistant penicillins, and aminopenicillins.
The group of extended-spectrum penicillins is composed of 2 different subgroups: a-carboxypenicillins (carbenicillin, ticarcillin) and acylaminopenicillins (piperacillin). a-Carboxypenicillins have a carboxylic acid group at the a-position at R on the penicillin nucleus and acylaminopenicillins have basic groups on the side chain at R on the penicillin nucleus.
Partly because of these polar groups, extended-spectrum penicillins are even more active against gram-negative aerobic and gram-negative anaerobic bacilli than are aminopenicillins, and use of extended-spectrum penicillins is generally limited to the treatment of serious infections caused by susceptible gram-negative bacilli or mixed aerobic-anaerobic bacterial infections. In vitro, extended-spectrum penicillins are generally active against gram-positive and gram-negative aerobic cocci that are susceptible to natural penicillins and aminopenicillins.
Like natural penicillins and aminopenicillins, extended-spectrum penicillins are hydrolyzed by staphylococcal penicillinases and are therefore inactive against penicillinase-producing strains of S. aureus and S. epidermidis.
Extended-spectrum penicillins have some activity against gram-positive aerobic and gram-positive anaerobic bacilli, but the drugs are generally less active in vitro on a weight basis against these organisms than are natural penicillins and aminopenicillins. Extended-spectrum penicillins are generally active in vitro against gram-negative bacilli that are susceptible to aminopenicillins.
The drugs are also active against many strains of Enterobacteriaceae and some strains of Pseudomonas that are resistant to other currently available penicillins. a-Carboxypenicillins are active in vitro against some strains of E. coli, Morganella morganii (formerly Proteus morganii), Proteus mirabilis, P. vulgaris, Providencia rettgeri, Salmonella, Shigella, and Ps. aeruginosa. In addition to these organisms, acylaminopenicillins are generally active in vitro against some strains of Citrobacter,Enterobacter, Klebsiella, and Serratia; however, there are some differences among the acylaminopenicillins in spectra of activity and levels of activity against these organisms. Extended-spectrum penicillins are generally more active in vitro against Bacteroides fragilis than other currently available penicillins.
Because clavulanic acid can inhibit certain b-lactamases that generally inactivate ticarcillin, combinations of ticarcillin disodium and clavulanate potassium are active in vitro against many b-lactamase-producing organisms that are resistant to ticarcillin alone. For more complete information on the spectra of activity of penicillins and additional information on the drugs, see the General Statements on Natural Penicillins, Aminopenicillins, Penicillinase-Resistant Penicillins, and Extended-Spectrum Penicillins and the individual monographs in 8:12.16.04, 8:12.16.08, 8:12.16.12, and 8:12.16.16.




