Support Drug Guide: buy high-quality generic medicines at our sponsor, the best pharmacy store online. You are welcome to place an order and get Stromectol (Ivermectin) 3mg, 6mg, 12mg online over the counter. Low price and quick worldwide delivery are guaranteed. Use discounts, and remember: the more you buy, the better the price for a pill.
Ivermectin, a dihydroavermectin B1, is an effective microfilaricide used in the treatment of strongyloides, scabies, and all types of filariasis except Dipalonema (Mansonella) perstans infections.
Over the past several years, ivermectin has shown excellent results in treating onchocerciasis, both in controlled studies and in the field, including use in the WHO-sponsored treatment program. This experience has provided a thorough picture of its adverse effects. The effective dosage is 50-200 micrograms/kg. After a single oral dose, skin microfilariae remain at low levels for up to nine months.
Ivermectin Observational Studies
Among the published studies, some have specifically sought to define the pattern of adverse effects. In one such study, although a single dose of the drug was combined in some patients with diethylcarbamazine, the adverse effect pattern was similar to that when ivermectin was used alone. There now seem to be some circumstances in which a single low dose of ivermectin is sufficient to have a prolonged effect; for example, in loiasis, a dose of 150 micrograms/kg resulted in a very much reduced level of microfilaria as much as a year later and seemed to eliminate the infestation entirely in more than half the users. If further work confirms the validity of this approach, the adverse reactions problem may be lessened since, at these doses, the few reactions experienced were limited to the skin and joints, although some calabar-like swellings were also noted.
Brugia Malayi
In an open study from India, 21 asymptomatic microfilaria carriers (with counts of 109-6934/ml of blood) were treated with a single oral dose of ivermectin 400 micrograms/kg and a single oral dose of diethylcarbamazine 6 mg/kg for infection with Brugia malayi. Twelve hours after treatment, microfilaria counts fell by 96-100% in all patients, and 12 patients had become afilaremic. All had an adverse reaction, lasting up to 48 hours after treatment: fever, myalgia, headache, lethargy, chills, sweating, anorexia, sore throat and pharyngeal congestion, arthralgia, giddiness, nausea and vomiting, abdominal pain, and cough. Postural hypotension, lasting 1 day, was noted in two individuals. Transient dilated and painfully inflamed lymphatic channels, which stood out in cords, were seen in two individuals. Most adverse effects were mild and self-limiting.
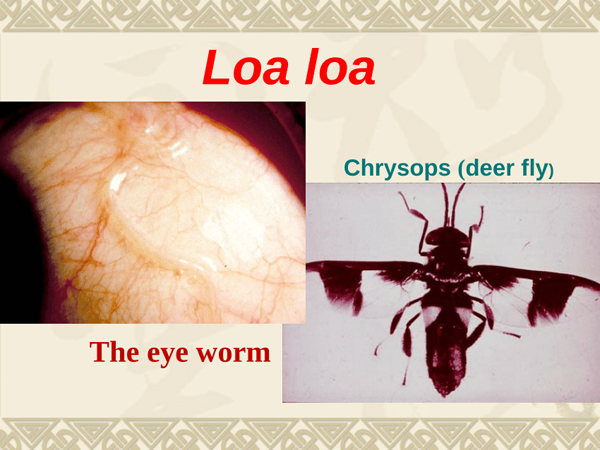
Loa Loa
The use of ivermectin and its adverse effects in patients infected with Loa loa have been reviewed. It was concluded that ivermectin in a single dose of 150-300 micrograms/kg is effective in reducing microfilaria counts by over 90% with suppressed counts to 25% of pretreatment values after 1 year. An even more sustained effect can be reached by more frequent dosing. There is also some evidence that ivermectin in higher doses (400 micrograms/kg twice yearly) can affect the adult Loa loa. Tolerance to ivermectin is generally excellent, but serious adverse effects, especially encephalopathy, can occur, principally in more heavily infected individuals.
Onchocerciasis
The impact of 5 years of annual community treatment with ivermectin on the prevalence of onchocerciasis and onchocerciasis-associated morbidity in the village of Gami (Central African Republic) has been assessed. Pruritus, onchocercal nodules, and impaired vision were all significantly reduced by annual treatment with ivermectin.
In a study of the effect of ivermectin on adult Onchocerca worms, the following regimens were compared:
- 150 micrograms/kg yearly (reference group);
- 400 micrograms/kg, then 800 micrograms/kg yearly;
- 150 micrograms/kg 3-monthly;
- 400 micrograms/kg, then 800 micrograms/kg 3-monthly.
After 3 years of treatment, more female worms had died in those who were treated every 3 months than in the reference group; female worms were also less fertile. There was no difference between the two groups of patients who were treated yearly.
There were no serious adverse events, even at high doses. However, subjective complaints of visual disturbances, such as blurred vision, ocular pain, or dyschromatopsia, were more frequent in those who were given 800 micrograms/kg than in those who were given 150 micrograms/kg; the effects lasted less than 1 week. Detailed ocular examination showed no differences between patients from the reference group and the three other groups.
Sarcoptes Scabiei
In an uncontrolled open study, 101 patients with scabies were treated with a single oral dose of ivermectin 200 micrograms/kg and then followed at 3 days and at 2 and 4 weeks. Two weeks after the start of treatment 89 patients were completely free of scabies, while another three had only mild lesions and pruritus with negative skin scrapings. The other nine patients had persistent pruritus and new lesions and were treated with a second dose, with a complete cure in all cases after 4 weeks.
Twelve patients reported minor adverse effects, consisting of drowsiness, arthralgia and bone aches, dyspnea, headache, nausea, and blurred vision. The adverse effects were mostly reported at the first follow-up and were easily tolerated. Ivermectin appears to be a safe and effective treatment for scabies in a dose of 200 micrograms/kg, although a second dose is necessary for a complete cure in a few patients.
An 11-year-old girl developed severe crusted Norwegian scabies. Gamma-benzene hexachloride lotion and topical keratolytics had no significant effect. She was given a single oral dose of ivermectin 6 mg/kg with dramatic effect. The pruritus subsided in 4 hours, and the lesions started to clear 2 days later. A second dose of 6 mg was given after 3 weeks when no skin lesions were found anymore. The only adverse effect was some edema of the skin after the first dose, which did not occur after the second dose, suggesting that the reaction was more related to the intensity of the infection than to the effect of the drug itself.
Outbreaks of scabies in elderly people require special management for disease control. Owing to the frequent failure of repeated non-synchronized therapeutic efforts with conventional external antiscabie treatments, special eradication programs are required.
The management of outbreaks of scabies with allethrin, permethrin, and ivermectin has been evaluated. Healthy infested people were treated once simultaneously with an external scabicide, such as allethrin or permethrin; this was effective in 99%. Those with crusted scabies were hospitalized and treated with systemic ivermectin or ivermectin plus permethrin; seven patients received ivermectin twice after an interval of 8 days, and one received permethrin three times. Unfortunately, no details of adverse effects were given.
Strongyloidiasis
The efficacy and adverse effects of ivermectin 200 micro-grams/kg, repeated 2 weeks later, have been studied in 50 patients with chronic strongyloidiasis, aged 30-79 years. The eradication rate was 96% at 2 weeks after the first dose and 98% after the second dose. There was no recurrence after a follow-up of 4 months. One patient had nausea and vomiting 3 hours after the first dose and again after the second dose, but they were transient and required no therapy. In four patients, there were mild laboratory abnormalities (slight increases in liver function tests in two, microscopic hematuria in one, and mild leukopenia and lymphocytosis in one). Of the 50 patients, 12 were positive for human T lymphotropic virus type-1.
Wuchereria Bancrofti
Early-stage elephantiasis caused by bancroftian filariasis in a 27-year-old traveler was treated with a single-dose oral combination of ivermectin 24 mg plus albendazole 400 mg, followed by albendazole 800 mg for 21 days. To avoid a severe Mazzotti-like reaction, he was given oral glucocorticoids and antihistamines for 3 days. He had a transient rash, pruritus, and mild hypotension on the days after the initial treatment but otherwise remained well, and the swelling subsided. Within 1 month, he was free of symptoms. At the last follow-up examination, 3 years after treatment, there was no clinical or laboratory evidence of relapse. The authors thought that this type of treatment should be evaluated on a wider scale, given the minimal adverse events and apparent therapeutic efficacy.
The efficacy of annual mass chemotherapy with a combination of diethylcarbamazine and ivermectin on bancroftian filariasis in rural southern India has been studied, as has the supplementary role of controlling the vector mosquito Culex quinquefasciatus. Nine villages, topographically and ecologically similar but reasonably isolated from each other, were selected and split into three comparable groups of three villages each.
Group A received chemotherapy with diethylcarbamazine at about 6 mg/kg and ivermectin at 400 micrograms/kg. Group B received chemotherapy and vector control. The most important vector-breeding sites were soakage pits, which were treated with expanded polystyrene beads. Minor vector-breeding sources, such as domestic or irrigation wells, were treated by adding larvae-eating Talapia fish or a commercial insecticide based on Bacillus sphaericus. Group C received no intervention.
After the first round of treatment, combination chemotherapy alone caused a 60% drop in the annual filarial transmission potential, whereas the combined strategy reduced the transmission potential by 96%. After two rounds of treatment, the reduction in transmission potential was similar to the two strategies (about 91-96% reduction), whereas the prevalence of microfilaremia was reduced by 88-92%.
Adverse events after combination therapy were reported in 20% of those who had taken diethylcarbamazine and ivermectin for the first time. The patients with adverse events had increased microfilarial counts. The most common adverse effects were headache (72% of adverse events), giddiness (67%), fever, and weakness. The incidence of adverse events among those taking combination therapy for a second time was relatively low (5.5%).
The adverse events were also less severe in the second round than in the first. When antifilarial treatment was withdrawn in the third and final year of the study, transmission was resumed in the absence of vector control, whereas no infective female mosquitoes were detected in villages with vector control. Vector control, although obviously not cost-effective in the short term, could therefore play an important supplementary role in an integrated program, by preventing re-establishment of transmission after chemotherapy has been completed.
Ivermectin Comparative Studies
Sarcoptes Scabiei
In a randomized trial, a single oral dose of ivermectin (200 micrograms/kg) was compared with 1% gamma-benzene hexachloride lotion for topical application overnight in 200 patients with scabies. The patients were assessed after 48 hours, 2 weeks, and 4 weeks. After 4 weeks, 83% showed marked improvement with ivermectin, compared with 44% of those treated with gamma-benzene hexachloride. There were no adverse events reported with gamma-benzene hexachloride. Headache was reported only once with ivermectin.
In 80 children aged 6 months to 14 years a single dose of ivermectin 200 micrograms/kg was compared with topical benzyl benzoate for the treatment of pediatric scabies in a randomized, controlled trial. Ivermectin cured 24 of 43 patients, and topical benzyl benzoate cured 19 of 37 patients at 3 weeks after treatment. There were no serious adverse effects with either treatment, although benzyl benzoate was more likely to produce local skin reactions. These results are in line with those of another study, in which 18 children aged 14 months to 17 years with either scabies or cutaneous larva migrans were treated with a single dose of ivermectin 150-200 micro-grams/kg. A single oral dose cured 15 patients, and three patients with crusted scabies required a second dose. None had significant adverse reactions.
Wuchereria Bancrofti
In a study in which doses of 200 or 400 micrograms were used, with or without diethylcarbamazine, for Wuchereria bancrofti infection, there was a higher than average incidence of reactions (and a higher incidence with ivermectin than with diethylcarbamazine), but this perhaps reflected an unusually high success rate or the severity of the original infection. For similar reasons, repeated courses of treatment tend to show a falling incidence of adverse effects. Normally, such general symptoms as fever, weakness, anorexia, malaise, and chills occur in a substantial minority of patients on a first course, while at least one-third have muscle and/or joint pains. Vertigo, dyspnea, diarrhea, and abdominal disturbances affect a few patients. The severity of adverse effects is not related to serum concentrations of the drug, which again reflects the fact that they are largely a consequence of the parasitic breakdown rather than the toxic effects of ivermectin.
Ivermectin Placebo-Controlled Studies
Emesis, ataxia, and mydriasis are cardinal signs of ivermectin toxicity. The safety, tolerability, and pharmacokinetics of escalating high-dose ivermectin have been studied in 68 healthy subjects in a randomized, double-blind, placebo-controlled study in the following doses:
- 30 mg fasted
- 60 mg fasted
- 90 mg fasted
- 120 mg fasted, 30 mg fed
Ivermectin was generally well tolerated. Quantitative pupillometry ruled out any mydriatic effect of ivermectin. There was no nervous system toxicity associated with oral ivermectin at any of the doses. There were no serious clinical or laboratory adverse events. Three of the fifty-one subjects who took ivermectin fasted reported minor adverse gastrointestinal events: fecal abnormality, nausea, and vomiting; six reported minor neurological adverse events: headache, anxiety, and dizziness. There were no adverse events in the subjects who took ivermectin 120 mg. The absorption of ivermectin was about 2.5 times higher when it was given after a high-fat meal.
Sarcoptes Scabiei
In a randomized, double-blind comparison of the efficacy of oral ivermectin and topical gamma-benzene hexachloride, 53 patients were randomly allocated to either a single oral dose of ivermectin 150-200 micrograms/kg and a placebo topical solution or a single dose of gamma-benzene hexachloride topical solution 1% and placebo tablets. Patients who did not fulfill the criteria for a clinical cure within 15 days, defined as the absence of both pruritus and clinical lesions or a reduction in signs and symptoms to a mild degree, repeated the initial treatment.
Of the 53 patients, 43 completed the study (19 of those treated with ivermectin and 24 of those treated with gamma-benzene hexachloride). After 15 days, 74% of the patients treated with ivermectin and 54% of the patients treated with gamma-benzene hexachloride were considered to be cured. At 29 days, both treatments were equally effective, with cure rates of 95% and 96%, respectively. Adverse effects were mild and transient in both groups. One of the patients treated with ivermectin had hypotension, one had abdominal pain, one had vomiting, and one complained of headache. There were no abnormalities on routine laboratory testing.
Wuchereria Bancrofti
In a double-blind, placebo-controlled study in Ghana, single doses of ivermectin 150-200 micrograms/kg and albendazole 400 mg, either separately or in combination, were given to 1425 individuals for Wuchereria bancrofti infection. Of these, 340 were microfilariae-positive before treatment. Ivermectin and ivermectin plus albendazole both produced statistically significant reductions in mean microfilaria counts at follow-up; the effect of ivermectin was longer lasting. Albendazole produced a non-significant reduction. Adverse reactions were few and mostly mild, and there were no severe reactions.
Mechanism of Action
The mode of action of ivermectin has been reviewed. It has tentatively been identified as agonism at GABA receptors, with inhibition of ion channels that control specific nerve cell connections. The functioning of chloride channels should thus be altered in most organisms, leading to paralysis and death of parasites. Several sites of action have been proposed:
- a postsynaptic agonist site either on the receptor or in its immediate neighborhood;
- a presynaptic site of activation of GABA release;
- potentiation of GABA binding to its receptor.
Another mechanism of action involves the binding of ivermectin to P glycoprotein.
Lactation
Only a small amount of ivermectin is excreted in breast milk, and it has been suggested that it is unnecessary to exclude lactating mothers from mass chemotherapy with ivermectin.
Side Effects
As antihelminthic drugs go, ivermectin can be considered a reasonably safe drug, and it is generally better tolerated than diethylcarbamazine. Clinical experience has often shown relatively little toxicity, although mild adverse effects, presumably due to the killing of the microfilariae, involve at least one-third of patients; some work has suggested that neutrophil activation may play a role in the development of these reactions. It has also been well tolerated in combinations, for example, when given with albendazole in order to kill adult worms (which cannot be achieved with ivermectin alone) or with diethylcarbamazine for bancroftian filariasis.
The principal reservation from the start was that ivermectin has a long half-life and that some late effects might occur in certain individuals. During the early phases, it was recommended that in areas where the drug had been widely used, the health workers involved should continue to observe patients for a time in case problems did arise, but no late complications have, in fact, been documented.
General Adverse Effects
Acute symptoms, often flu-like or affecting the skin, are related almost entirely to the release of toxic products and allergens from the killed filariae and can affect two-thirds of patients; in conditions in which this type of reaction does not occur, one may suspect that the drug is ineffective. The mechanism of the effects also explains why they tend to occur early and sometimes briefly, that is, immediately after the microfilariae die. For similar reasons, these effects are most severe in patients with a high microfilaria count.
Despite the sometimes transient and apparently tolerable nature of the skin effects, they can persist in patients requiring long-term treatment, for example, for onchocerciasis, and under these conditions, they are sufficient to impair compliance with treatment.
The effects of age, sex, dosing round, time of day, and distance from the nurse monitor on adverse event reporting during mass ivermectin administration in Achi in South-East Nigeria have been examined. There was a significant increase in adverse reporting with age, but not sex. Fewer adverse effects were reported after starting at night than after starting by day. There was no significant effect of distances up to 1 km on adverse events reporting. Both compliance and adverse reporting were less after the second dosing round than after the first. These variables should be included in the standardization of adverse events reporting.
Ivermectin Side Effects in Treating Onchocerciasis
Although adverse reactions after ivermectin in onchocerciasis are usually less severe than after diethylcarbamazine, they still affect a significant number of patients with onchocerciasis after the first dose. With subsequent treatments, these reactions become less frequent and severe. The so-called Mazzotti reaction, which is often seen after treatment of Onchocerca volvulus with diethylcarbamazine or ivermectin, is characterized by fever, tachycardia, hypotension, adenitis, pruritus, arthralgia, a papular or urticarial rash, and lymphedema. It is ascribed to an inflammatory host response to microfilaria killing and tends to be more severe in those who have greater numbers of parasites. The roles of chemoattractants, such as eotaxin, RANTES, and MCP-3, in the recruitment of eosinophils to the site of the parasite killing, have been studied in 13 patients with onchocerciasis and two control subjects before and after ivermectin. There were adverse reactions in eight patients, but none were severe. The reactions were fever (54%), pruritus (62%), rash (46%), and lymphedema (46%). There was no significant postural hypotension. There was endothelial expression of both RANTES and eotaxin after ivermectin, suggesting that these chemoattractants have an important role in eosinophil recruitment into the skin during the killing or degeneration of parasites after ivermectin.
A role for the release of Wolbachia bacterial endosymbionts has been suggested in the pathogenesis of the Mazzotti reaction. There was a good correlation between Wolbachia DNA, serum TNF-alfa, and the antibacterial peptides calprotectin and calgranulin after treatment with ivermectin or diethylcarbamazine, supporting a role for Wolbachia products in mediating these inflammatory responses.
There has been an epidemiological survey of the endemicity of human onchocerciasis and the effects of subsequent mass distribution of ivermectin in villages of the Nzerem-Ikpem community in Nigeria. Of 1126 people studied, 527 were positive for skin microfilariae, 329 had leopard skin (characterized by focal skin depigmentation), 385 had nodules, and 167 had acrodermatitis. There were adverse effects in 362 patients (19%): pruritus in 13%, limb swelling in 8.5%, facial swelling in 2%, weakness in 4.8%, nausea and vomiting in 3.4%, headache in 5.8%, diarrhea in 3.4%, and rheumatism in 3.5%. There were no severe reactions.
Ivermectin Side Effects in Treating Loa Loa Encephalopathy
When treating Loa loa infections on a large scale with ivermectin, the encephalopathy that was a much-feared complication with diethylcarbamazine again seems to occur, especially with heavily infected or older individuals.
For this reason, mass use of ivermectin in areas of endemic Loa loa infection is no longer recommended. Ivermectin appears to promote the passage of Loa loa microfilaria into the cerebrospinal fluid, with a maximum after 3-5 days, followed by an intense allergic reaction to the dying microfilaria. The Mectizan Expert Committee defined a definite case of Loa loa encephalopathy related to ivermectin as having to satisfy two criteria:
- encephalopathy in which there is microscopic evidence of vasculopathy in the brain associated with Loa loa microfilaria;
- the onset of symptoms of disturbed nervous system function within 5 days after treatment with ivermectin, progressing to coma without remission.
A probable case of Loa loa encephalopathy was defined as having to satisfy four criteria:
- coma in a previously healthy individual;
- the onset of nervous system signs within 5 days of treatment with ivermectin progressing to coma;
- an initial microfilaremia of over 10 000/ml, or 1000/ml in a blood sample taken within 2 months of treatment;
- the presence of Loa loa microfilaria in the CSF.
Clinically common features of this condition are impaired consciousness appearing 3-4 days after treatment and lasting for 2-3 days.
There is no consensus on the proper management of ivermectin-associated Loa loa encephalopathy, and it is uncertain if co-administration of glucocorticoids is of any use. In several patients with more severe reactions, conjunctival hemorrhages were seen.
A systematic examination of the conjunctivae in 1682 patients complaining of any adverse reactions showed that these hemorrhages were closely correlated with the pretreatment microfilaria counts. This sign can be found 2 days after treatment and may thus single out patients susceptible to encephalopathy and needing closer follow-up. Although the incidence of such cases is very low (in the order of 1 in 10 000 treated patients), this serious adverse effect makes mass treatment of Loa loa infection problematic, and also mass treatment of onchocerciasis in areas in which Loa loa is endemic. To illustrate this point, three probable cases of Loa loa encephalopathy after ivermectin treatment for onchocerciasis have been described. All three were young men treated with ivermectin 150 micrograms/kg in a mass-treatment campaign in onchocerciasis.
- A 26-year-old previously healthy man developed nervous system symptoms in the form of an inability to stand or eat and stiffness of the neck by the third day. On the fourth day, he had difficulty swallowing and speaking. On the fifth day, he could not speak and was incontinent of urine. He was given dexamethasone, diazepam, furosemide, and atropine. On the sixth day, he became comatose. On the ninth day, he developed a high fever and was given penicillin and tube-feeding. His condition gradually worsened, and he died on the 21st day. Serum microfilaria counts on day 13 after treatment were still high (3600/ml), and live Loa loa (10/ml) were found in the CSF.
- A 32-year-old man with alcoholism had a very high pretreatment serum microfilaria count (50 000/ml). After starting ivermectin, he took to his bed and would not speak. On the third day, he developed a fever, possibly attributed to malaria, and was treated with chloroquine. On the fourth day, he was unable to stand and alternately restless or somnolent; his CSF contained live Loa loa microfilaria. He became more incoherent and fidgety and had a marked grasp reflex. Later in the day, he developed spastic hypertonia. On the fifth day, he became incontinent and still would not speak. Over the following days, he gradually improved and, 4 months later, had no neurological abnormalities, although his relatives found that his behavior had changed and that he was much calmer than in the past. An electroencephalogram on day 15 showed periodic diffuse discharges of large amplitude during hyperventilation, and on day 146, an asymmetric tracing with focal activity in the right parieto-occipital area, which worsened during hyperventilation. On day 233, the electroencephalogram was normal.
- An 18-year-old previously healthy man was given ivermectin. On the second day, he was unable to work and stayed at home. On the third day, he was found unconscious in bed, incontinent of urine and feces. On the fourth day, he did not move and had absent pain sensation. There was hypertonia in the arms with marked cogwheeling. On the fifth and sixth days, there was a swinging horizontal movement of the eyeballs, but otherwise, he appeared to improve. On the seventh day he could stay seated in bed with help and spoke several sentences. He could perform slow voluntary movements, and his muscle strength and sensation returned to normal, although the cogwheel phenomenon still persisted. He gradually returned to normal over the following weeks. After 5 months, the neurological examination was normal, but he still complained of headaches and episodic amnesia. His pretreatment serum microfilaria counts were high (152 940/ml), and the CSF collected on the fourth day contained live Loa loa microfilaria. An electroencephalogram on the 19th day was slow with spontaneous, diffuse, paroxysmal, monomorphic theta activity, lasting 2-3 seconds. An electroencephalogram on the 105th day showed improvement, but focal abnormalities persisted in the left occipital region. On the 159th day all previously recorded abnormalities had disappeared.
Side Effects on Organs and Systems
Cardiovascular
Supine and postural tachycardia with postural hypotension can occur; in one large study, such effects were found in three of 40 patients. In another, hypotension was found in 13 of 69 cases, but in some series, these effects have not been observed at all. A massive community study in Ghana noted hypotension in only 37 of nearly 15,000 patients treated. Transient electrocardiographic changes are sometimes seen.
Respiratory
In the treatment of Wuchereria bancroftian filariasis, respiratory capacity was evaluated in 23 patients with single doses up to 200 micrograms/kg; there was a transient but significant fall in vital capacity some 24-30 hours after administration, apparently due to bronchodilatation. Frank dyspnea occurred in 2% of cases in the study cited above. In other studies, a few patients developed a transient cough, and in others, pneumonitic patches were seen in the chest X-ray.
Nervous System
Headache and vertigo are very common and even usual as part of the flu-like reaction to ivermectin.
A puzzling reaction was recorded in a small hospitalized Canadian population of elderly subjects treated for scabies with a single dose of ivermectin (150-200 micrograms/kg). Within 6 months, 15 of the 47 patients had died. All those who died had developed a sudden change in behavior, with lethargy, anorexia, and listlessness before death. The effect may have been an artefact with some extraneous cause, and it is notable that other groups using this treatment for scabies have not recorded similar reactions.
Sensory Systems
Careful ophthalmological examination shows a striking increase in the number of microfilariae in the anterior chamber of the eye in a significant minority of patients, and a new inflammatory infiltrate can appear during treatment in already damaged areas of the retina. However, no permanent ocular sequelae have been documented. Most of the other ophthalmic symptoms, including edema and local inflammation, are those of the primary infection.
Conjunctival hemorrhages have been recorded in patients living in areas in which loiasis is endemic. Although ivermectin is usually well tolerated, these patients had serious adverse reactions after taking ivermectin, including an encephalopathy similar to that seen after treatment with diethylcarbamazine. In retrospect, these cases all had high Loa loa microfilaremia and Loa loa microfilariae in the cerebrospinal fluid. The authors suggested that ivermectin might have provoked the passage of Loa loa microfilariae into the cerebrospinal fluid. In a subsequent study of 1682 patients with loiasis treated with ivermectin 150 micrograms/kg, conjunctival hemorrhages were found in 41, nine of whom had previously received a microfilaricidal drug. The initial mean Loa loa microfilaremia was 14 900 microfilariae/ml (range 0-182 400; median, compared with 14.5 microfilariae/ml (range 0-97 600; median in those without conjunctival hemorrhage. In addition, male sex and Dipalonema perstans microfilaremia were associated with conjunctival hemorrhages. There was a close relation between conjunctival hemorrhages and retinal lesions. Based on observations in three patients who all developed coma after ivermectin, the authors suggested that retinal lesions may reflect what occurs in the cerebral circulation in patients with high Loa loa microfilaremia and neurological problems after ivermectin.
Hematologic
When 28 Sudanese patients were treated with a single dose of ivermectin for onchocerciasis, they developed a prolonged prothrombin time, which continued to lengthen significantly during the next 4 weeks; there were no changes in other clotting parameters. After a month, two of them developed hematomas, which continued to enlarge for the next 3^1 days. Both of these patients had received ivermectin 150 micrograms/kg. One was given a transfusion; the swellings in both cases resolved within a week. For a time, it was considered that the prothrombin changes observed in some such cases were a potential problem; more recent work suggests that the prolongation of prothrombin ratio is, in fact, hardly more than with placebo and that, in fact, ivermectin merely has a mild effect on vitamin K metabolism and little effect on coagulation. However, lymphadenitis has been noted in a few patients. In one Guatemalan study of biannual treatment of the population to eradicate Onchocerca volvulus infection, upper limb edema was noted in nearly 20% of cases receiving the treatment for the first time.
Gastrointestinal
In the late stages of Strongyloides hyperinfection, ileus can develop and hamper the absorption of oral medication.
A 39-year-old Afro-Caribbean man with stage IVB T cell lymphoma due to HTLV-1 infection had invasive Strongyloides hyperinfection that did not respond to oral ivermectin plus albendazole because of concurrent ileus. He was treated with two 6 mg doses of a veterinary formulation of ivermectin subcutaneously. There were no adverse effects, apart from pain at the injection site.
Urinary Tract
Proteinuria is unusual but has been described; it was detected 14 days after a single dose and disappeared during follow-up.
Observations that proteinuria and hematuria may occur in patients with filariasis bancrofti and loiasis, which may exacerbate after treatment with diethylcarbamazine or ivermectin, led to the study of kidney function in patients with onchocerciasis before and after treatment with ivermectin.
The occurrence of renal abnormalities was studied in a population-based study in a meso-endemic village (40% microfilaria carriers), in a group of patients with a generalized or hyper-reactive form of onchocerciasis, and in 46 patients treated with ivermectin in a single oral dose of 150 micrograms/kg. All individuals in all three study groups were examined clinically and had skin snips, serological testing for onchocerciasis, and nodulectomy (when relevant). Tests for malaria, schistosomiasis, intestinal nematodes, and hepatitis B, and serum glucose, creatinine, IgE, and electrophoresis were also performed.
The urine was tested for erythrocytes, leukocytes, protein, nitrites, pH, glucose, ketone bodies, urobilinogen, and creatinine. All the patients underwent renal ultrasound examination. There was no difference in renal function and renal ultrasound between patients with and without onchocerciasis. A raised urinary protein concentration (over 70 mg/g of creatinine) was common and occurred in 47% of the patients with onchocerciasis and 63% of the patients without onchocerciasis. In the 46 patients treated for onchocerciasis with a single dose of 150 micrograms/kg there was a slight but statistically significant increase in total urine protein after 2 and 5 days, especially in 16 patients with high pretreatment skin microfilaria counts. The abnormalities were minor and insignificant. Neither onchocerciasis itself nor treatment with ivermectin was associated with abnormalities of renal function.
Skin
A degree of pruritus, soreness, or burning sensation is common with ivermectin, and rashes or skin edema can occur, while pre-existing conditions of this type can be aggravated. The skin over hematomas can be discolored. Swelling of the limbs and face, like the dermatological symptoms, is probably a reaction to the breakdown products of the helminth.
Patients with severe skin involvement (“sowda”) as a facet of their onchocerciasis can experience transient aggravation of the condition, but the course is favorable, and they are less likely to have the same problem if it is later necessary to repeat treatment.
Rashes and swelling of the lymph nodes seem to be more common in patients with AIDS who take ivermectin.
Musculoskeletal
Joint or bone pains are common but usually mild; in one study myalgia occurred in 33% of cases and arthralgia in 33%.
Reproductive System
Orchitis or epididymitis with scrotal tenderness occurs in a few patients as a manifestation of the acute reaction as the parasite succumbs.



 (582 votes, average: 3.49 out of 5)
(582 votes, average: 3.49 out of 5)






How do you determine the exact dosage of Ivermectin . . . I know its by body weight, but how many milligrams would a 145 pound person take and how many milligrams would a 250 pound man take.
I understand taking the Ivermectin in two doses a week apart. This is for bird mites
Please consult with your doctor, or other qualified healthcare professional. We do not provide medical advice.